Copyright
©The Author(s) 2023.
World J Gastroenterol. Dec 7, 2023; 29(45): 5974-5987
Published online Dec 7, 2023. doi: 10.3748/wjg.v29.i45.5974
Published online Dec 7, 2023. doi: 10.3748/wjg.v29.i45.5974
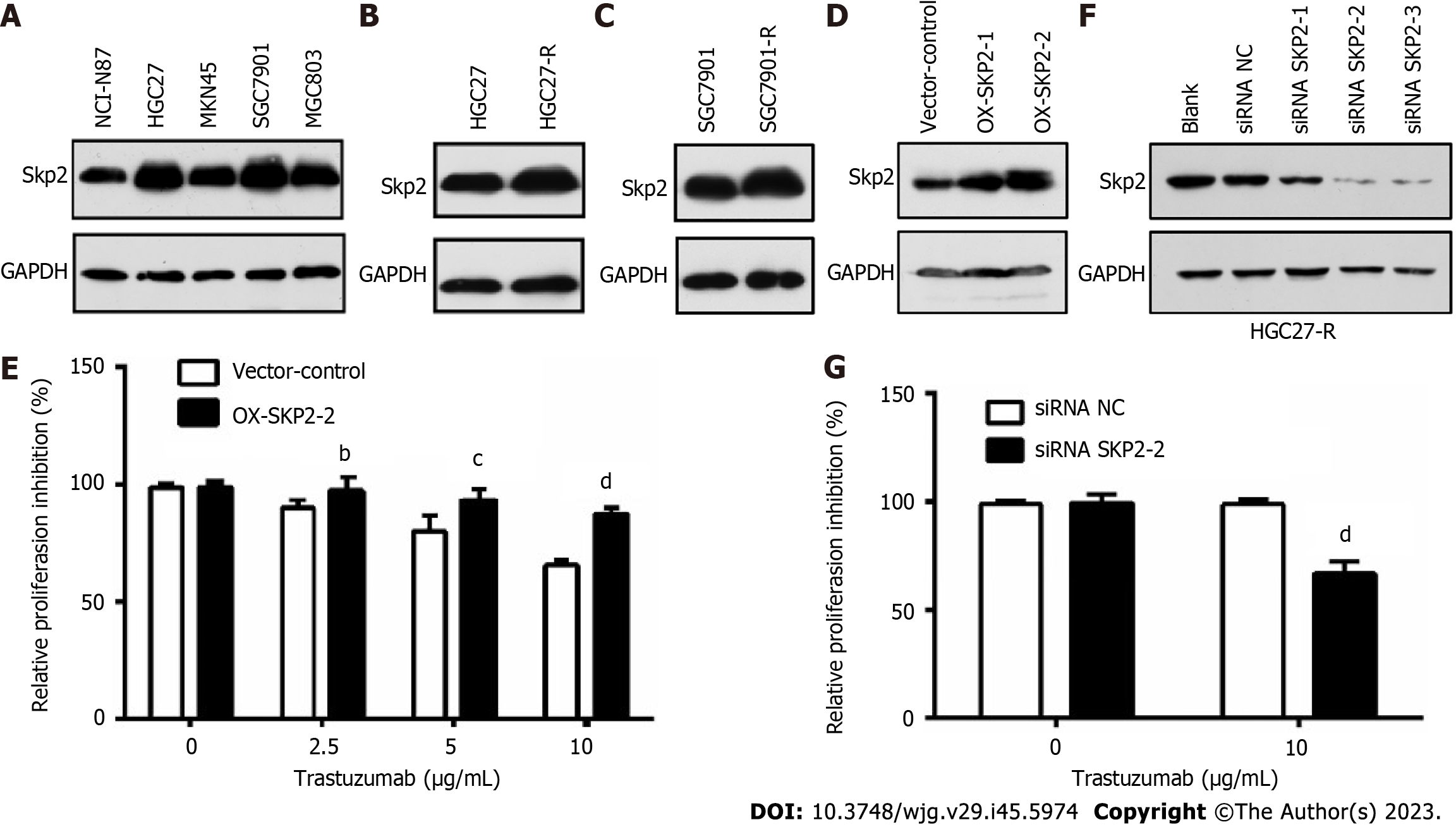
Figure 1 Elevated S-phase kinase associated protein 2 expression contributes to trastuzumab resistance in human epidermal growth factor receptor 2-positive gastric cancer cells.
A: Western blotting was used to measure the S-phase kinase associated protein 2 (Skp2) expression level in human gastric cancer (GC) cell lines; B and C: Skp2 expression in HGC27 and HGC27-R cells and SGC7901 and SGC7901-R cells was analyzed by Western blotting; D: Skp2 expression levels in HGC27 cells transfected with empty vector (pcDNA3.1), OX-SKP2-1 or OX-SKP2-2 (pcDNA3.1/SKP2) were determined by western blotting; E: HGC27 cells stably transfected with empty vector or OX-SKP2-2 were cultured with or without trastuzumab (0, 2.5, 5, and 10 μg/mL) for 48 h. Proliferation activity was analyzed by cell counting kit-8 (CCK-8) assays; F: HGC27-R cells were transfected with or without small interfering RNA-negative control (siRNA-NC) or siRNA-SKP2 for 72 h. Skp2 expression was measured by western blotting; G: HGC27 cells transfected with siRNA-NC or siRNA-SKP2-2 were cultured with or without 10 μg/mL trastuzumab. CCK-8 assays were used to analyze proliferation activity. The experiments were performed in triplicate. bP < 0.01; cP < 0.001; dP < 0.0001. Skp2: S-phase kinase associated protein 2; siRNA: Small interfering RNA; NC: Negative control.
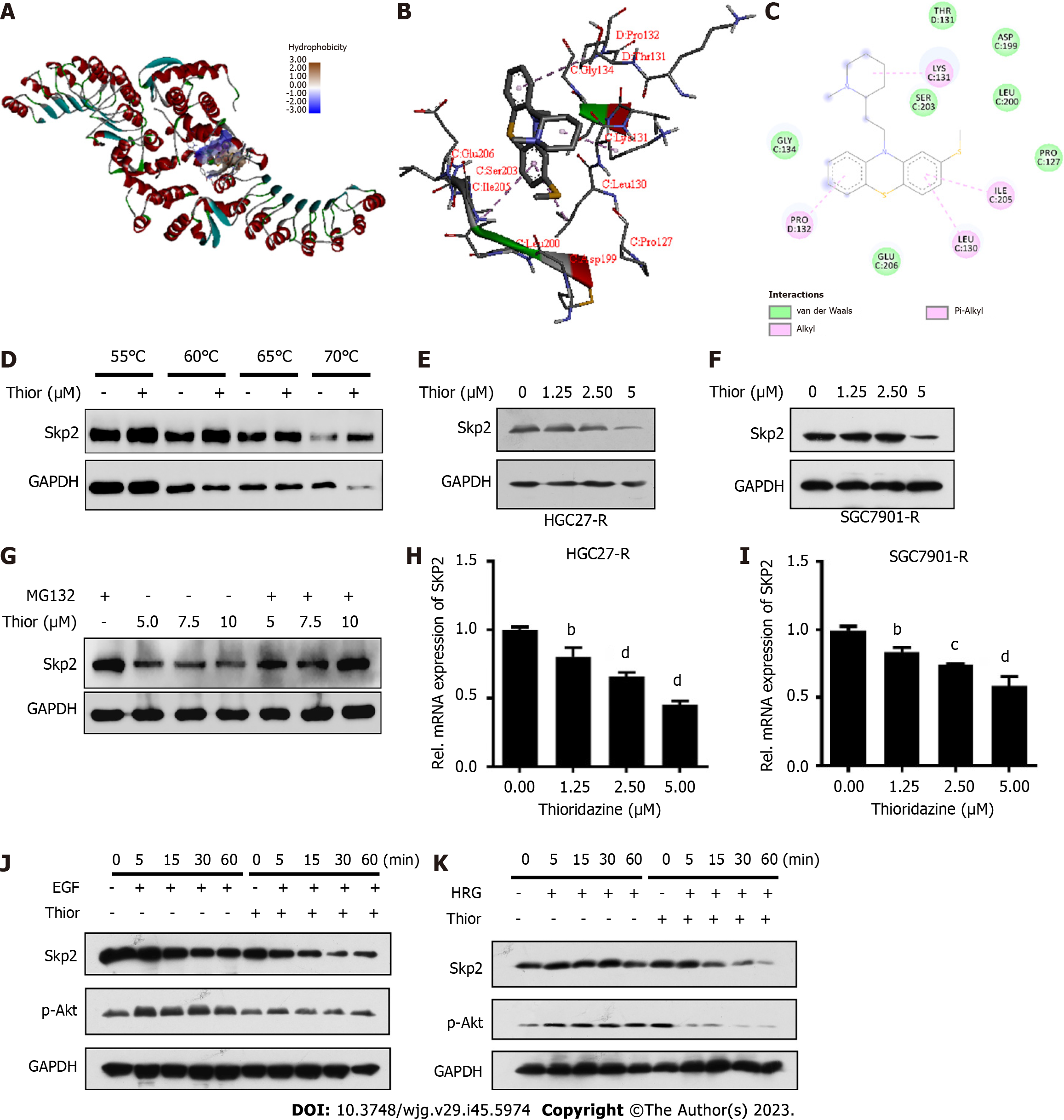
Figure 2 Thioridazine can inhibit S-phase kinase associated protein 2 expression and protein kinase B activation triggered by ErbB signaling.
A: The interaction between the ligand thioridazine and the active pocket of S-phase kinase associated protein 2 (Skp2) (1FS2) was examined; B: 3D structural schematic showing the binding of thioridazine to Skp2; C: Dihedral angle diagram showing the mode of interaction between thioridazine and Skp2; D: HGC-27-R cells were exposed for 24 h to dimethyl sulfoxide or 30 µM thioridazine and subjected to the Cellular Thermal Shift Assay; E and F: HGC27-R and SGC7901-R cells were treated with different concentrations of thioridazine for 24 h. Western blotting was used to analyze Skp2 expression; G: HGC27 cells were treated with the indicated concentrations for 42 h and were then treated with 5 μM MG132 for 6 h. The protein expression level of Skp2 was analyzed by western blotting; H and I: HGC27-R and SGC7901-R cells were treated with various concentrations of thioridazine for 24 h. The mRNA expression level of SKP2 was analyzed by Q-PCR; J and K: HGC27-R cells were pretreated with or without thioridazine (5 μM) for 2 h, followed by stimulation with epidermal growth factor (100 μg/mL) or heregulin (50 μg/mL). At the indicated times, Skp2 and p-protein kinase B protein levels were assessed. bP < 0.01; cP < 0.001; dP < 0.0001. Thior: Thioridazine; Skp2: S-phase kinase associated protein 2; EGF: Epidermal growth factor; HRG: Heregulin.
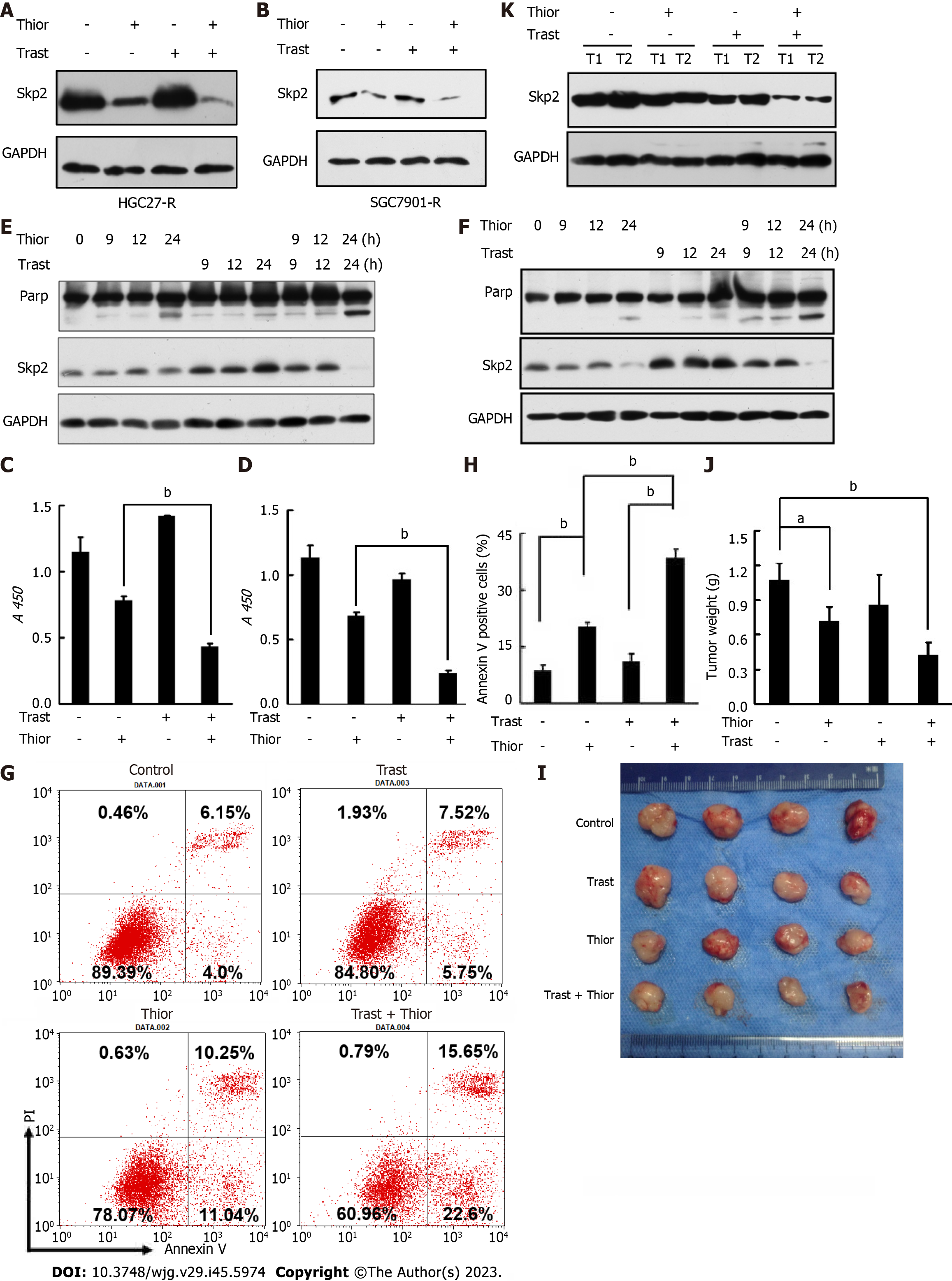
Figure 3 Thioridazine increases the primary susceptibility of gastric cancer cells to trastuzumab in vitro and in vivo.
A and B: HGC27-R and SGC7901-R cells were treated with phosphate-buffered saline (PBS), thioridazine (5 μM), trastuzumab (5 μg/mL), or thioridazine (5 μM) plus trastuzumab (5 μg/mL) for 24 h. S-phase kinase associated protein 2 (Skp2) expression was determined by western blotting; C and D: HGC27 and SGC7901 cells were treated with trastuzumab (10 μg/mL) or thioridazine (2.5 μM) alone or in combination for 48 h. Proliferation activity was evaluated by cell counting kit-8 assays; E and F: HGC27 and SGC7901 cells were treated with trastuzumab (10 μg/mL) or thioridazine (2.5 μM) alone or in combination for various times. The expression of Skp2 and Parp was analyzed by western blotting; G and H: HGC27 cells were treated with trastuzumab (10 μg/mL) or thioridazine (2.5 μM) alone or in combination for 24 h, followed by an apoptosis assay, quantitative results; I-K: Nude mice were injected s.c. with 0.1 mL of an HGC27 cell suspension (3 × 107 cells/mL) in the right upper flank. The mice were treated with PBS, trastuzumab (0.5 mg/kg), thioridazine (25 mg/kg), or trastuzumab (0.5 mg/kg) plus thioridazine (25 mg/kg) daily by intraperitoneal (i.p.) injection beginning 7 d after cell implantation. After two weeks of drug administration, the mice were sacrificed. Primary tumors were excised, photographed, and weighed. Western blot analysis was used to assess Skp2 expression in primary tumors. aP < 0.05; bP < 0.01. Thior: Thioridazine; Trast: Trastuzumab.
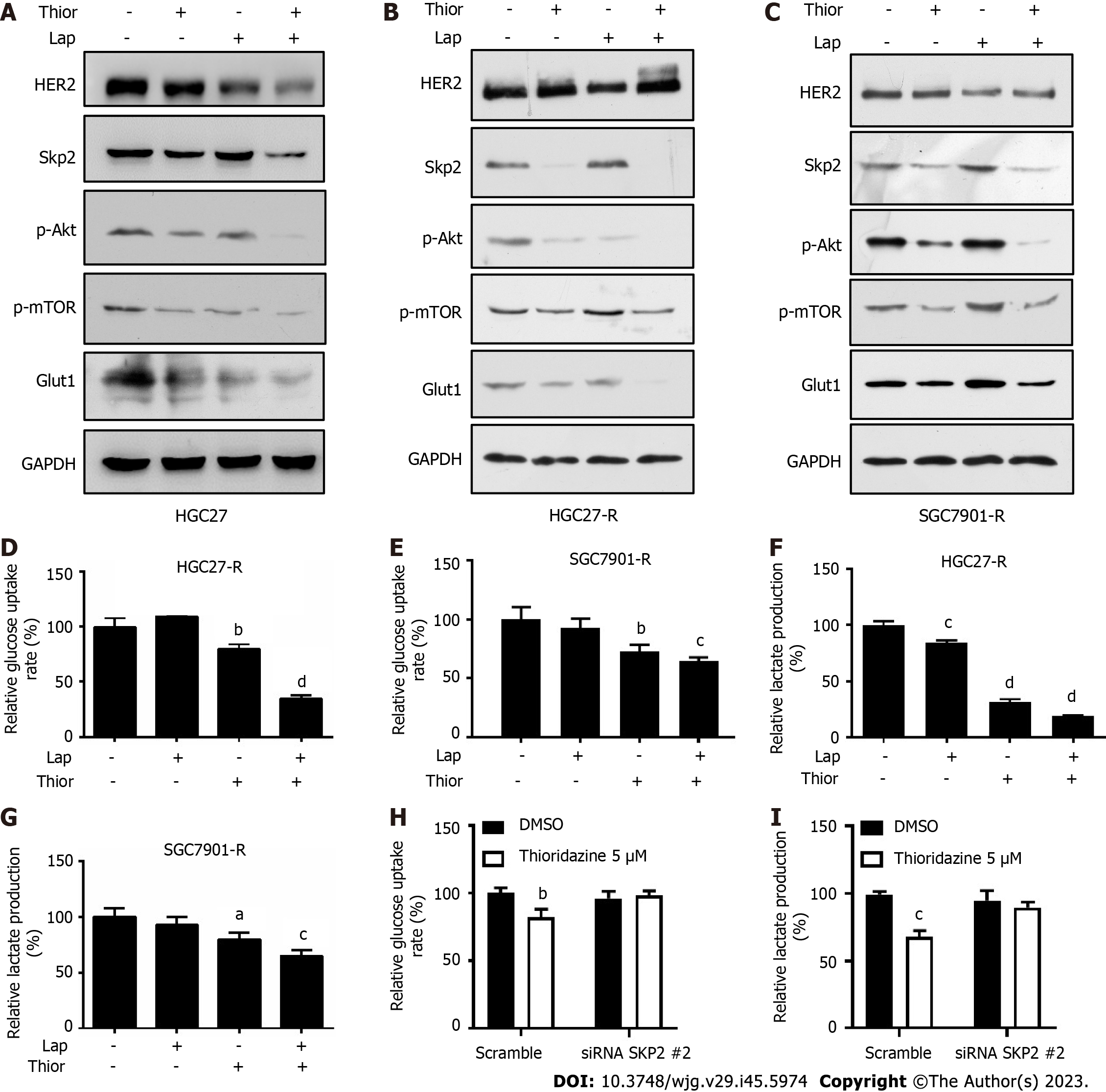
Figure 4 Thioridazine combined with lapatinib decreases S-phase kinase associated protein 2/p-protein kinase B/p-mammalian target of rapamycin/glucose transporter type 1 protein levels and glycolysis in gastric cancer cells.
A-C: HGC27, HGC27-R and SGC7901-R cells were treated with thioridazine (5 μM) alone or in combination with lapatinib (5 μM) for 24 h. S-phase kinase associated protein 2 (Skp2), p-protein kinase B (Akt), Akt, p-mammalian target of rapamycin, and glucose transporter type 1 protein levels were analyzed by western blotting; D-G: HGC27-R cells were treated with thioridazine (5 μM) and lapatinib (5 μM) alone or in combination for 24 h. The glucose uptake rate and lactate production rate were measured following the manufacturer's instructions; H and I: HGC27-R cells were divided into two groups that were transfected with small interfering RNA (siRNA) negative control or siRNA SKP2 2 for 48 h. Each group was treated without or with thioridazine (5 μM) for another 24 h. The glucose uptake rate and lactate production rate in all groups were measured. aP < 0.05; bP < 0.01; cP < 0.001; dP < 0.0001. Thior: Thioridazine; Lap: Lapatinib.
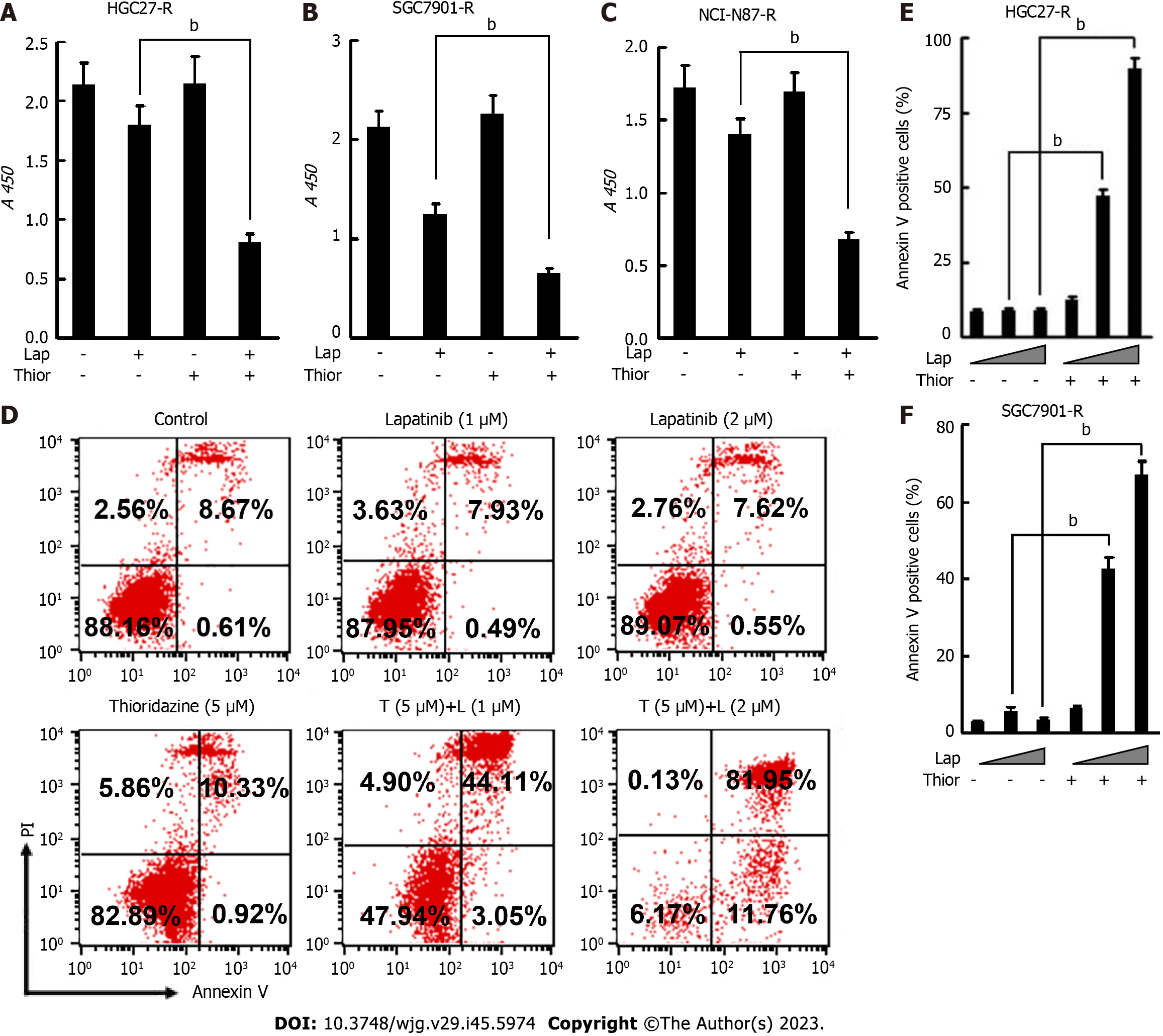
Figure 5 The combination of thioridazine and lapatinib exhibits more pronounced antitumor activity than either alone in vitro against trastuzumab-resistant gastric cancercells.
A-C: To HGC27-R, SGC7901-R, and NCI-N87-R cells were treated with trastuzumab (10 μg/mL) or thioridazine (5 μM) alone or in combination for 48 h. Proliferation activity was evaluated by cell counting kit-8 assays; D-F: HGC27-R cells were treated with lapatinib (1 μM or 2 μM) in the presence or absence of thioridazine (5 μM) for 24 h, followed by an apoptosis assay. Similar experiments were performed and results were quantified in SGC7901-R cells. The experiments were repeated three times. bP < 0.01. Thior: Thioridazine; Lap: Lapatinib.
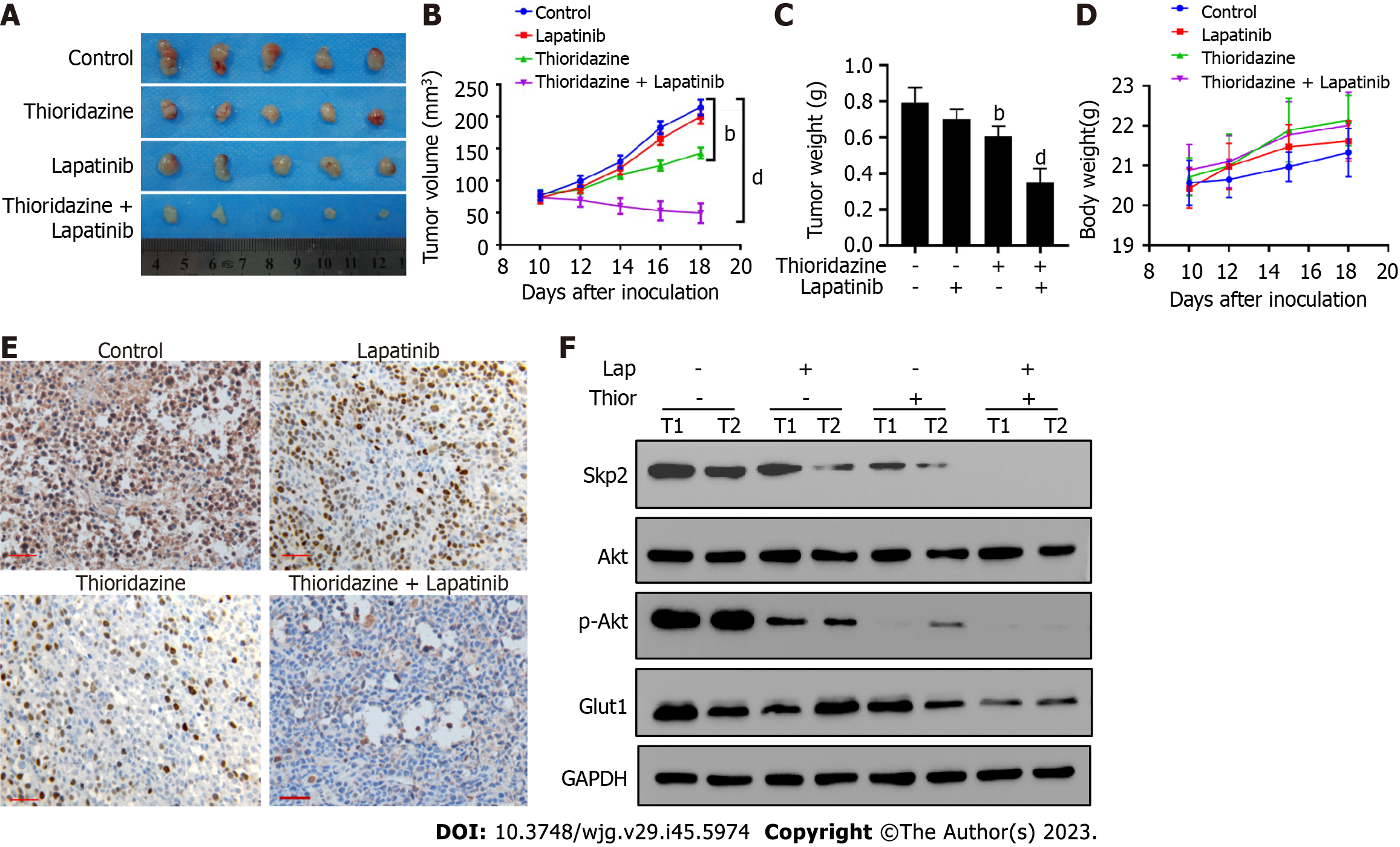
Figure 6 Coadministration of thioridazine and lapatinib demonstrates potent anticancer activity in mice harboring gastric cancer xenografts.
A: SGC7901-R cells (1 × 106) were injected s.c. into the flanks of nude mice. Ten days post-implantation, the mice were randomized into four groups of five mice each. Then, the mice were treated by i.p. injection with phosphate-buffered saline, lapatinib (70 mg/kg), thioridazine (25 mg/kg), or lapatinib (70 mg/kg) plus thioridazine (25 mg/kg) for 14 d. Gross morphology of the final excised xenograft tumor masses; B: Tumor volumes following treatments were recorded; C: Wet tumor weights; D: Body weights of mice; E: S-phase kinase associated protein 2 (Skp2) expression was evaluated by immunohistochemistry in primary tumors (scale bar = 100 μm); F: The protein levels of Skp2, protein kinase B (Akt), p-Akt, glucose transporter type 1, and GAPDH in two primary tumors from each group were measured by western blotting. bP < 0.01, dP < 0.0001. Thior: Thioridazine; Lap: Lapatinib.
- Citation: Yang ZY, Zhao YW, Xue JR, Guo R, Zhao Z, Liu HD, Ren ZG, Shi M. Thioridazine reverses trastuzumab resistance in gastric cancer by inhibiting S-phase kinase associated protein 2-mediated aerobic glycolysis. World J Gastroenterol 2023; 29(45): 5974-5987
- URL: https://www.wjgnet.com/1007-9327/full/v29/i45/5974.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i45.5974