Copyright
©The Author(s) 2023.
World J Gastroenterol. Aug 7, 2023; 29(29): 4499-4527
Published online Aug 7, 2023. doi: 10.3748/wjg.v29.i29.4499
Published online Aug 7, 2023. doi: 10.3748/wjg.v29.i29.4499
Figure 1 Illustration of the pathway of glucose metabolism.
Glucose is taken up by cells and undergoes a series of reactions to convert it to pyruvate via the process of glycolysis. Pyruvate can then enter the tricarboxylic acid cycle in the mitochondria to produce energy, or it can be converted to lactate in the cytosol under anaerobic conditions. The key enzymes involved in these reactions are highlighted in pale-purple, and linked pathways are depicted in pale-green. The mitochondrial complexes that are critical for oxidative phosphorylation and adenosine triphosphate production are shown in pale-blue. GLUT: Glucose transporter; HK: Hexokinase; G6P: Glucose-6-phosphate; G6PI: Glucose-6-phosphate isomerase; F6P: Fructose-6-phosphate; NADPH: Nicotinamide adenine dinucleotide phosphate; PFK1: Phosphofructokinase-1; F2,6BP: Fructose-2,6-bisphosphate; PFKBP3: Fructose-2,6-biphosphatase 3; F1,6BP: Fructose-1,6-bisphosphate; G3P: Glyceraldehyde-3-phosphate; DHAP: Dihydroxyacetone phosphate; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase; 1,3BPG: 1,3-bisphosphoglycerate; 3PG: 3-phosphoglycerate; PGK: Phosphoglycerate kinase; PGAM: Phosphoglycerate mutase; 2PG: 2-phosphoglycerate; ENO: Enolase; PEP: Phosphoenolpyruvate; PKM1/2: Pyruvate kinase isozyme M1/M2; LDH: Lactate dehydrogenase; MCT: Monocarboxylate transporter family; PDH: Pyruvate dehydrogenase; IDH: Isocitrate dehydrogenase; α-KG: α-ketoglutarate; OAA: Oxaloacetate; SDH: Succinate dehydrogenase; FH: Fumarate hydratase; I: Mitochondrial complex I; II: Mitochondrial complex II; III: Mitochondrial complex III; IV: Mitochondrial complex IV; V: Mitochondrial complex V; Q: Co-enzyme Q; cyto C: Cytochrome c; ATP: Adenosine triphosphate; ADP: Adenosine diphosphate; FADH2: Flavin adenine dinucleotide; e-: Electrons.
Figure 2 The complex interplay between glycolysis and oxidative phosphorylation in cancer cells.
This figure highlights the signaling networks and metabolic regulation in both Warburg-like and oxidative cancer cells. p53 induces PTEN and represses PI3K activity, which inhibits glycolysis and opposes the Warburg effect. Hypoxia and the subsequent activation of hypoxia-inducible factor 1 (HIF-1) play a crucial role in modulating various aspects of cancer cell metabolism, including glycolysis, lactate production, and the tricarboxylic acid (TCA) cycle. Hypoxia counteracts the degradation of HIF-1 by prolyl hydroxylases and von Hippel-Lindau, which stabilizes and activates HIF-1. HIF-1 then transcriptionally activates genes such as hexokinase, phosphofructokinase-1, aldolase A, PGK1, PGAM1, ENO1, and LDHA, as indicated by the red arrows. During glycolysis, excessive lactate can be exported to the extracellular environment, leading to microenvironmental changes such as a lower pH. Intracellular lactate can also be transferred to adjacent cells and re-converted to pyruvate, which can enter the TCA cycle and drive oxidative phosphorylation in oxidative cancer cells. GLUT: Glucose transporter; HK: Hexokinase; G6P: Glucose-6-phosphate; G6PI: Glucose-6-phosphate isomerase; F6P: Fructose-6-phosphate; NADPH: Nicotinamide adenine dinucleotide phosphate; PFK1: Phosphofructokinase-1; F2,6BP: Fructose-2,6-bisphosphate; PFKBP3: Fructose-2,6-biphosphatase 3; F1,6BP: Fructose-1,6-bisphosphate; G3P: Glyceraldehyde-3-phosphate; DHAP: Dihydroxyacetone phosphate; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase; 1,3BPG: 1,3-bisphosphoglycerate; 3PG: 3-phosphoglycerate; PGK: Phosphoglycerate kinase; PGAM: Phosphoglycerate mutase; 2PG: 2-phosphoglycerate; ENO: Enolase; PEP: Phosphoenolpyruvate; PKM1/2: Pyruvate kinase isozyme M1/M2; LDH: Lactate dehydrogenase; MCT: Monocarboxylate transporter family; PDH: Pyruvate dehydrogenase; IDH: Isocitrate dehydrogenase; α-KG: α-ketoglutarate; OAA: Oxaloacetate; SDH: Succinate dehydrogenase; FH: Fumarate hydratase; I: Mitochondrial complex I; II: Mitochondrial complex II; III: Mitochondrial complex III; IV: Mitochondrial complex IV; V: Mitochondrial complex V; Q: Co-enzyme Q; cyto C: Cytochrome c; HIF-1: Hypoxia-inducible factor 1; PHD: Prolyl hydroxylases; VHL: Von Hippel-Lindau.
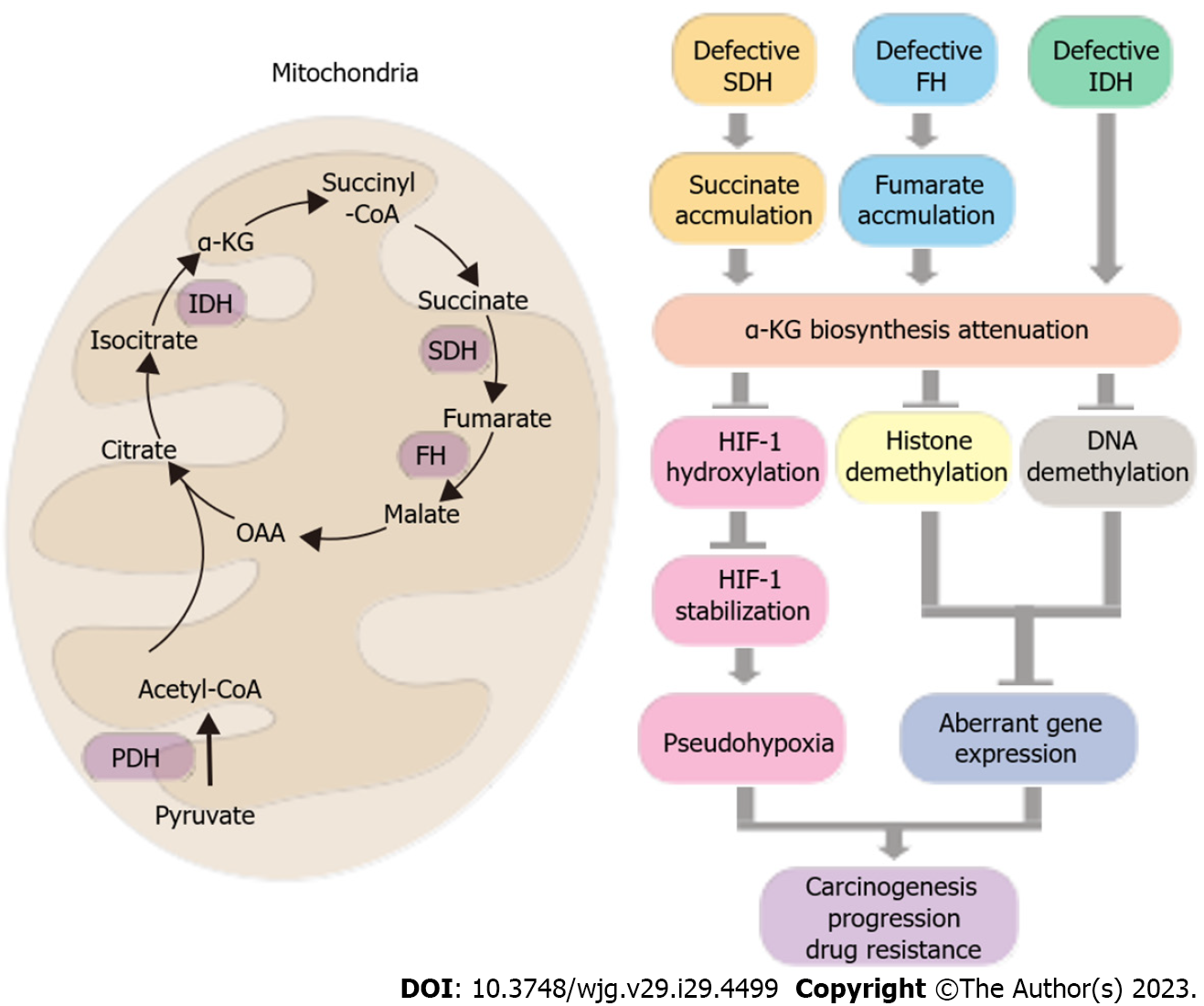
Figure 3 Tricarboxylic acid cycle dysfunction in cancer and its role in carcinogenesis, progression, and anti-cancer drug resistance.
The left panel depicts the tricarboxylic acid cycle, with succinate dehydrogenase and fumarate hydratase as key regulatory enzymes responsible for the formation of oncometabolites succinate and fumarate. The isocitrate dehydrogenase enzyme synthesizes α-ketoglutarate, which serves as a substrate for tumor suppressor pathways, such as hypoxia-inducible factor 1 hydroxylation for degradation, as well as histone and DNA demethylation. These processes can lead to pseudohypoxia and aberrant gene expression, promoting carcinogenesis, progression, and anti-cancer drug resistance. The right panel provides a summary of these relationships. OAA: Oxaloacetate; SDH: Succinate dehydrogenase; FH: Fumarate hydratase; PDH: Pyruvate dehydrogenase; IDH: Isocitrate dehydrogenase; α-KG: α-ketoglutarate; HIF-1: Hypoxia-inducible factor 1.
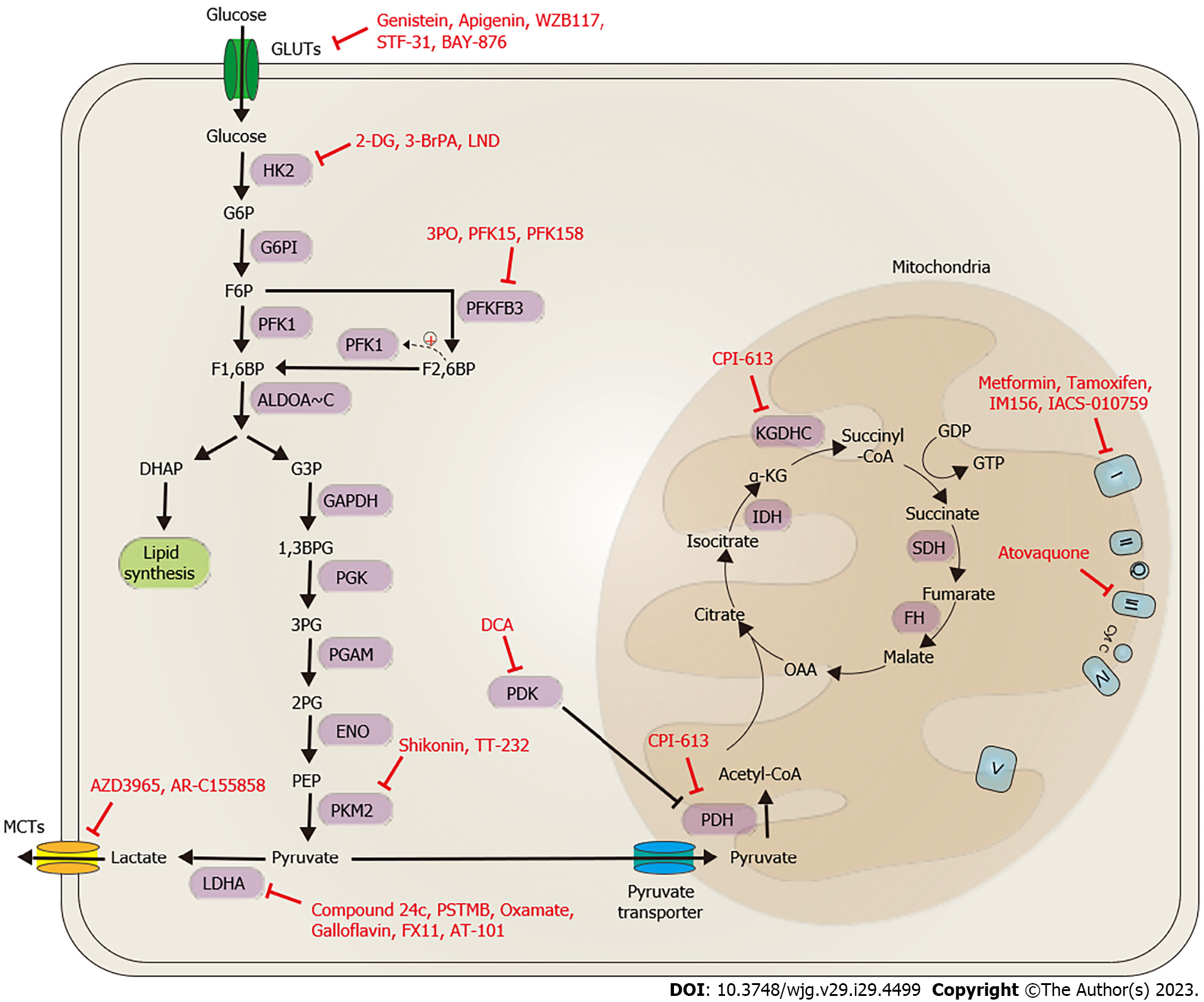
Figure 4 Potent bioenergetic-targeting drugs for gastrointestinal cancers.
Promising bioenergetic drugs for gastrointestinal cancers can be classified into two main categories based on their mode of action. The first category involves targeting aerobic glycolysis and lactate biosynthesis/transportation, while the second category involves targeting the tricarboxylic acid (TCA) cycle and oxidative phosphorylation (OXPHOS). Strategies to target aerobic glycolysis include blocking glucose importation through the targeting of glucose transporter 1 with compounds such as genistein, apigenin, WZB117, STF-31, and BAY-876, reducing glycolysis activity by targeting HK2 with compounds such as 2-DG, 3-BrPA, and LND, and targeting PKMFB3 and PKM2 with compounds such as 3PO, PFK15, PFK158, shikonin, and TT-232. Lactate biosynthesis can be inhibited by targeting LDHA with compounds such as compound 24c, PSTMB, oxamate, galloflavin, FX11, and AT-101, and PDK with DCA. Lactate transportation can be blocked by targeting MCT1/2 with compounds such as AZD3965 and AR-C155858. Targeting the TCA cycle and OXPHOS involves using inhibitors of pyruvate dehydrogenase, such as CPI-613, and mitochondrial complex I with metformin, tamoxifen, IM156, IACS-010759, and complex III with atovaquone. GLUT: Glucose transporter; HK: Hexokinase; G6P: Glucose-6-phosphate; G6PI: Glucose-6-phosphate isomerase; F6P: Fructose-6-phosphate; NADPH: Nicotinamide adenine dinucleotide phosphate; PFK1: Phosphofructokinase-1; F2,6BP: Fructose-2,6-bisphosphate; PFKBP3: Fructose-2,6-biphosphatase 3; F1,6BP: Fructose-1,6-bisphosphate; G3P: Glyceraldehyde-3-phosphate; DHAP: Dihydroxyacetone phosphate; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase; 1,3BPG: 1,3-bisphosphoglycerate; 3PG: 3-phosphoglycerate; PGK: Phosphoglycerate kinase; PGAM: Phosphoglycerate mutase; 2PG: 2-phosphoglycerate; ENO: Enolase; PEP: Phosphoenolpyruvate; PKM1/2: Pyruvate kinase isozyme M1/M2; LDH: Lactate dehydrogenase; MCT: Monocarboxylate transporter family; PDH: Pyruvate dehydrogenase; IDH: Isocitrate dehydrogenase; α-KG: α-ketoglutarate; OAA: Oxaloacetate; SDH: Succinate dehydrogenase; FH: Fumarate hydratase; I: Mitochondrial complex I; II: Mitochondrial complex II; III: Mitochondrial complex III; IV: Mitochondrial complex IV; V: Mitochondrial complex V; Q: Co-enzyme Q; cyto C: Cytochrome c; KGDHC: α-ketoglutarate dehydrogenase complex.
- Citation: Chu YD, Chen CW, Lai MW, Lim SN, Lin WR. Bioenergetic alteration in gastrointestinal cancers: The good, the bad and the ugly. World J Gastroenterol 2023; 29(29): 4499-4527
- URL: https://www.wjgnet.com/1007-9327/full/v29/i29/4499.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i29.4499