Copyright
©The Author(s) 2023.
World J Gastroenterol. May 14, 2023; 29(18): 2733-2746
Published online May 14, 2023. doi: 10.3748/wjg.v29.i18.2733
Published online May 14, 2023. doi: 10.3748/wjg.v29.i18.2733
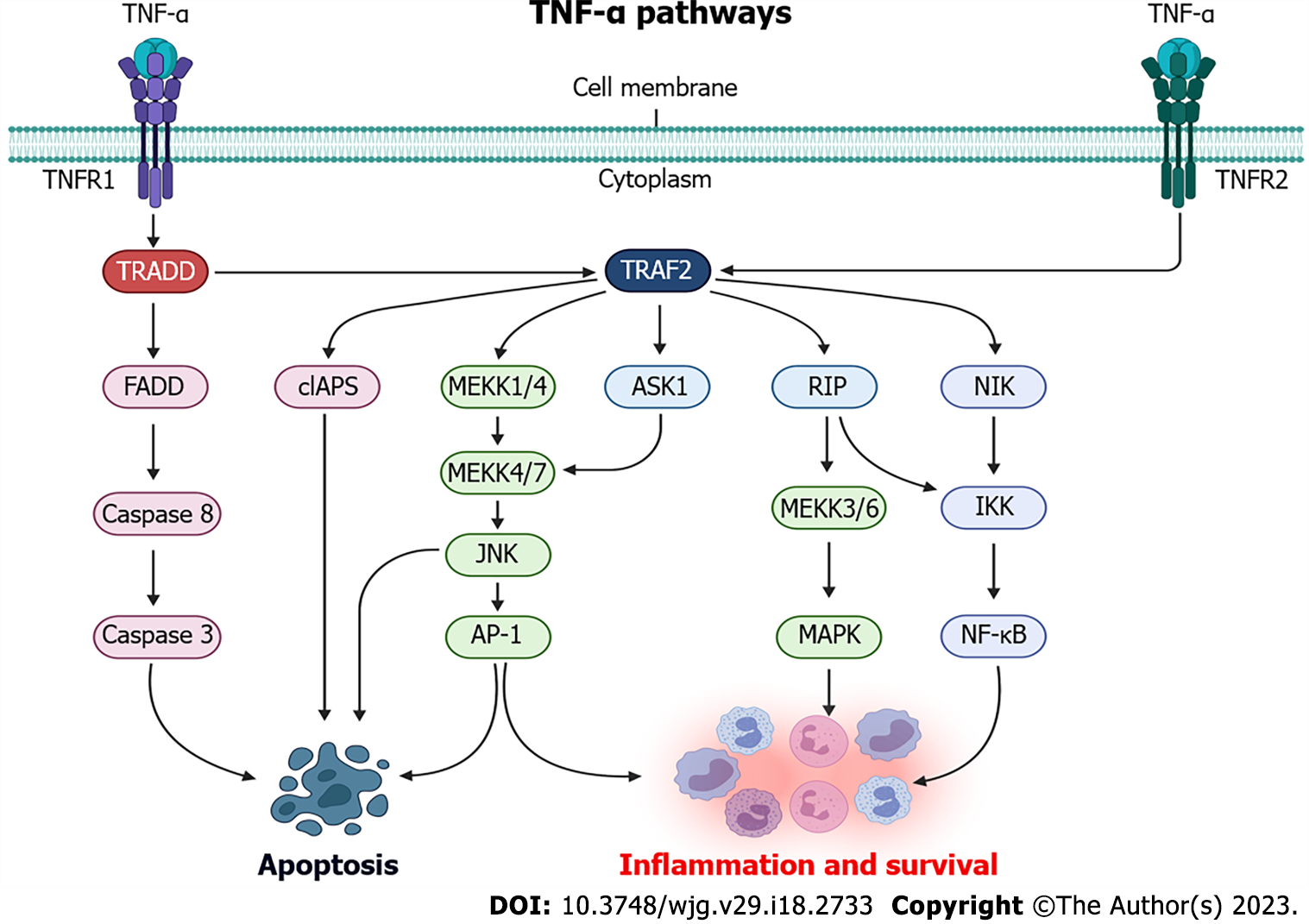
Figure 1 Tumor necrosis factor-alpha signaling pathways.
TNF-α: Tumor necrosis factor-alpha; TNFR1: Tumor necrosis factor receptor 1; TNFR2: Tumor necrosis factor receptor 2; TRADD: TNF receptor associated protein with death domain; FADD: FAS-associated death domain protein; cIAPS: Cellular inhibitor of apoptosis; MEKK: MAPK kinase kinas; JNK: N-terminal jun kinase; ASK1: Apoptosis signal-regulating kinase 1; IKK: IκB kinase; NF-κB: Nuclear factor κB; RIP: Receptor-interacting protein kinase. Created with BioRender.com.
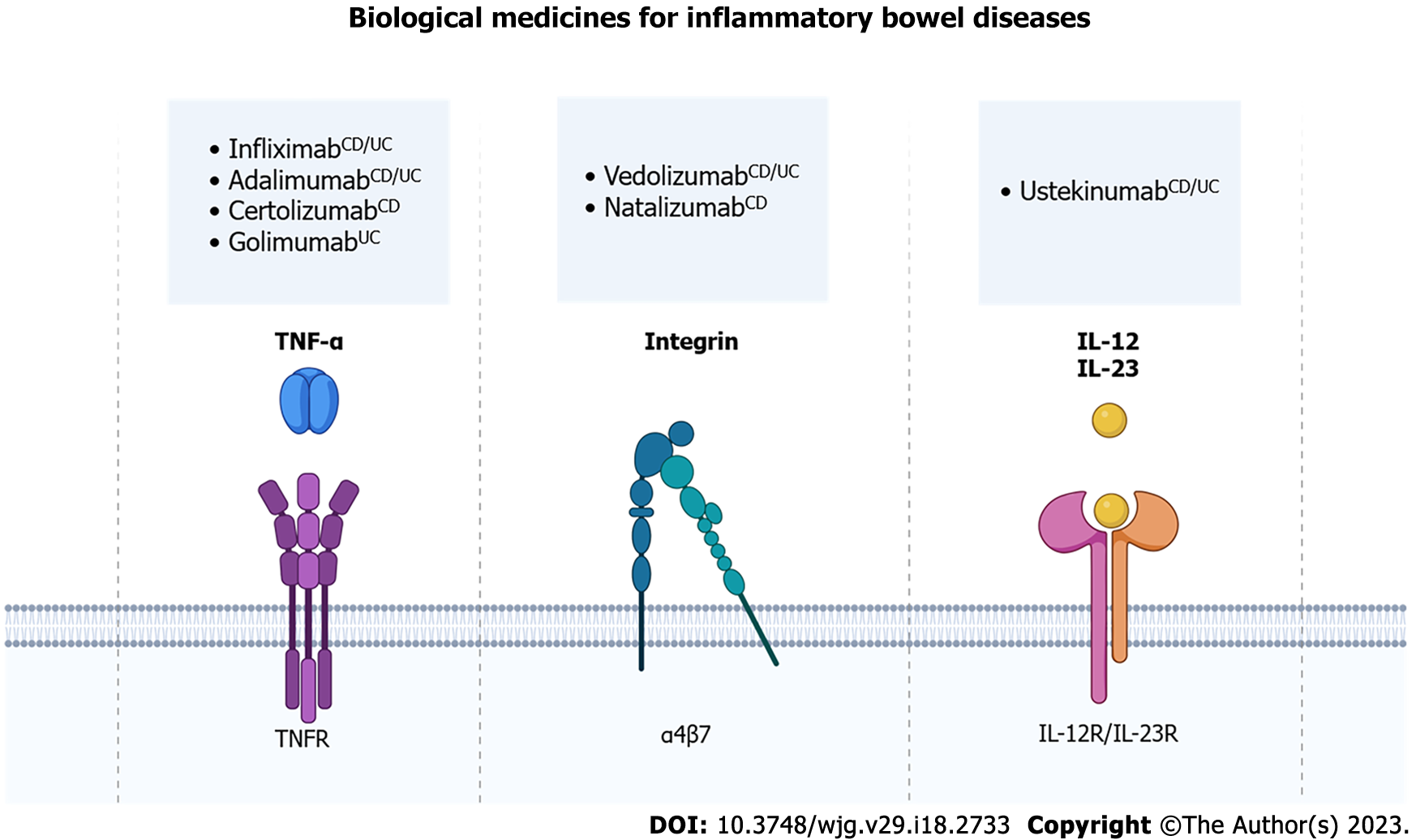
Figure 2 Biological medicines for inflammatory bowel diseases.
Biological medicines used for the treatment of inflammatory bowel diseases (IBD) can be classified into anti- Tumor necrosis factor (TNF)-α, anti-integrin and anti-interleukin therapies. Anti-TNF-α binds to TNF-α and inhibits TNF receptor activation. Infliximab, adalimumab, certolizumab pegol and golimumab are examples of anti-TNF-α used in the treatment of IBD. Vedolizumab and natalizumab are anti-integrin agents, which bind to the α4β7 integrin and prevent the migration of inflammatory cells into the intestinal tissue. Ustekinumab is an anti-interleukin that blocks interleukin (IL)-12 and IL-23, which cannot bind to IL-12 receptor and IL-23 receptor on T and B lymphocytes, thus reducing the inflammatory response in the gut. CDindication only for Crohn’s Disease; UCindication only for Ulcerative Colitis; CD/UCindication both for Crohn’s Disease and Ulcerative Colitis. TNF-α: Tumor necrosis factor-alpha; IL: Interleukin. Created with BioRender.com.
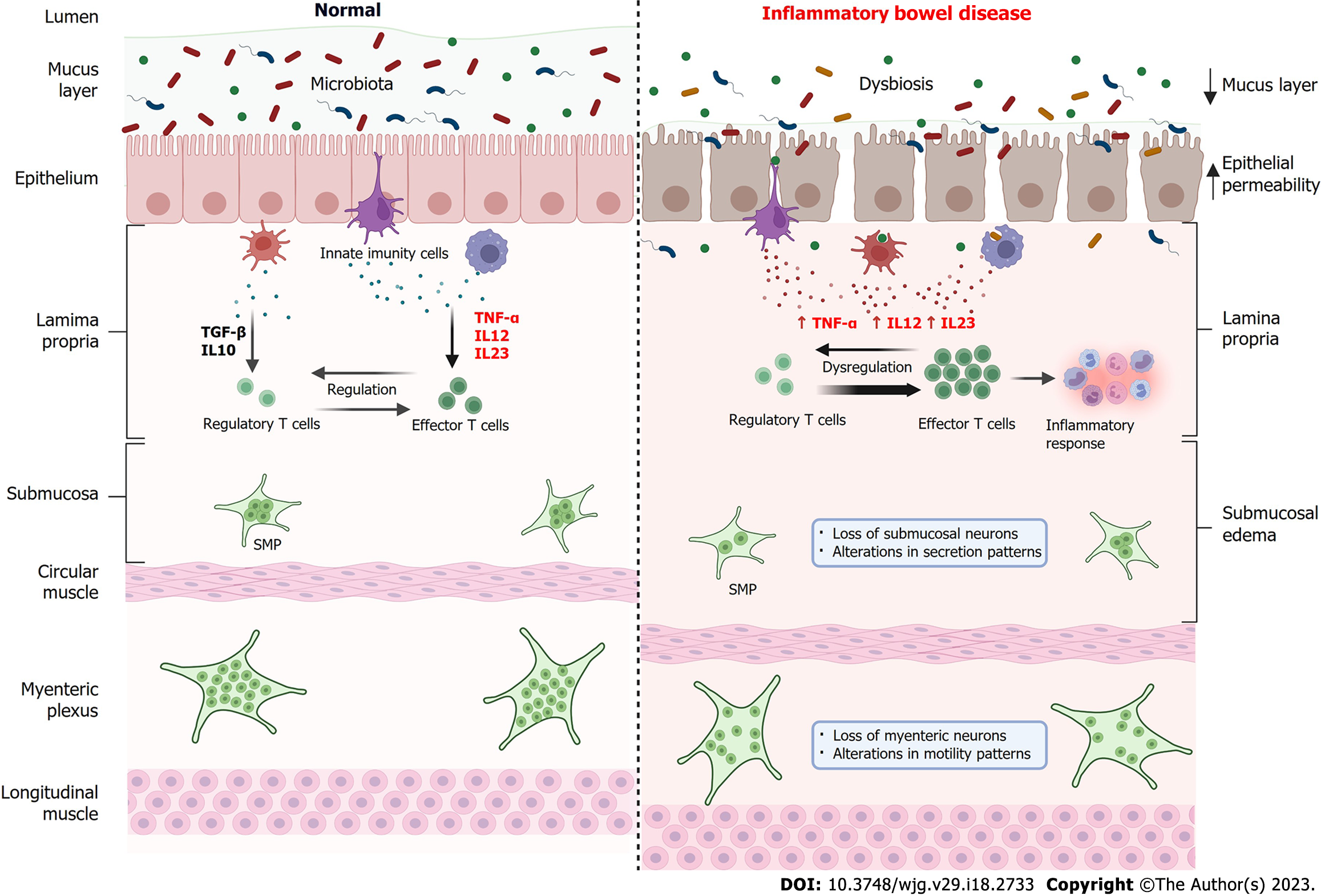
Figure 3 Comparison of the intestine in normal conditions and in the bowel with inflammatory bowel disease.
Under normal conditions (left), the intestinal microbiota establishes a symbiotic condition with the host, the intestinal barrier is intact and functional, and there is a balance between the levels of pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α), interleukin (IL)-12 and IL-23 and anti-inflammatory cytokines such as transforming growth factor beta and IL-10 by innate immune cells. In this way, there is a regulation of the immune response through a balance between regulatory T cells and effector T cells. Submucosal plexus (SMP) and myenteric plexus neurons are functional and controlling, respectively, fluid secretion and intestinal motility. In inflammatory bowel diseases (IBD) (right), there is an imbalance in the intestinal microbiota, the intestinal barrier is compromised, with a reduction in the mucous layer, increased epithelial permeability and consequent passage of microorganisms to the lamina propria. Innate immune cells increase the secretion of pro-inflammatory cytokines such as TNF-α, IL-12 and IL-23, which leads to a dysregulation of the immune system mediated by T cells, increasing the activity of effector T cells which, in turn, recruit cells for the inflammatory response. In IBD, submucosal edema is observed, as well as a reduction in the number of neurons in the SMP, causing changes in secretion patterns and loss of neurons in the myenteric plexus, resulting in changes in motility patterns. TNF-α: Tumor necrosis factor-alpha; IL: Interleukin. Created with BioRender.com.
- Citation: Souza RF, Caetano MAF, Magalhães HIR, Castelucci P. Study of tumor necrosis factor receptor in the inflammatory bowel disease. World J Gastroenterol 2023; 29(18): 2733-2746
- URL: https://www.wjgnet.com/1007-9327/full/v29/i18/2733.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i18.2733