Copyright
©The Author(s) 2023.
World J Gastroenterol. Mar 14, 2023; 29(10): 1614-1626
Published online Mar 14, 2023. doi: 10.3748/wjg.v29.i10.1614
Published online Mar 14, 2023. doi: 10.3748/wjg.v29.i10.1614
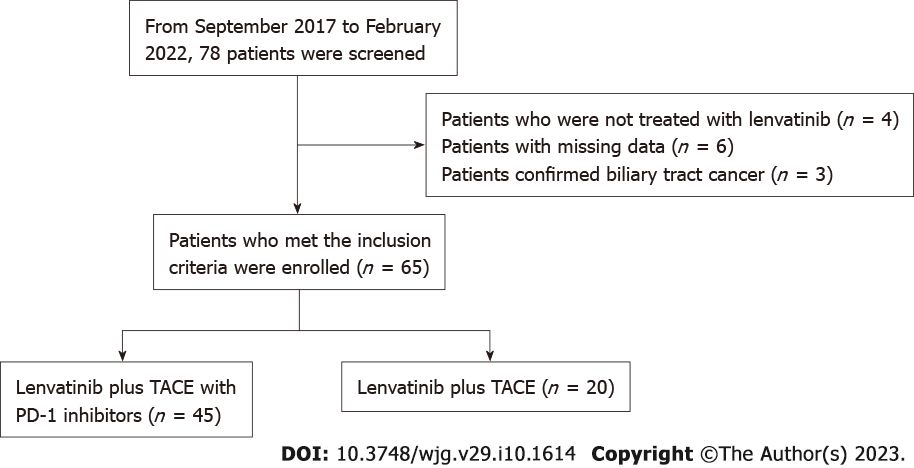
Figure 1 Workflow of this study.
TACE: Transarterial chemoembolization; PD-1: Programmed death receptor-1.
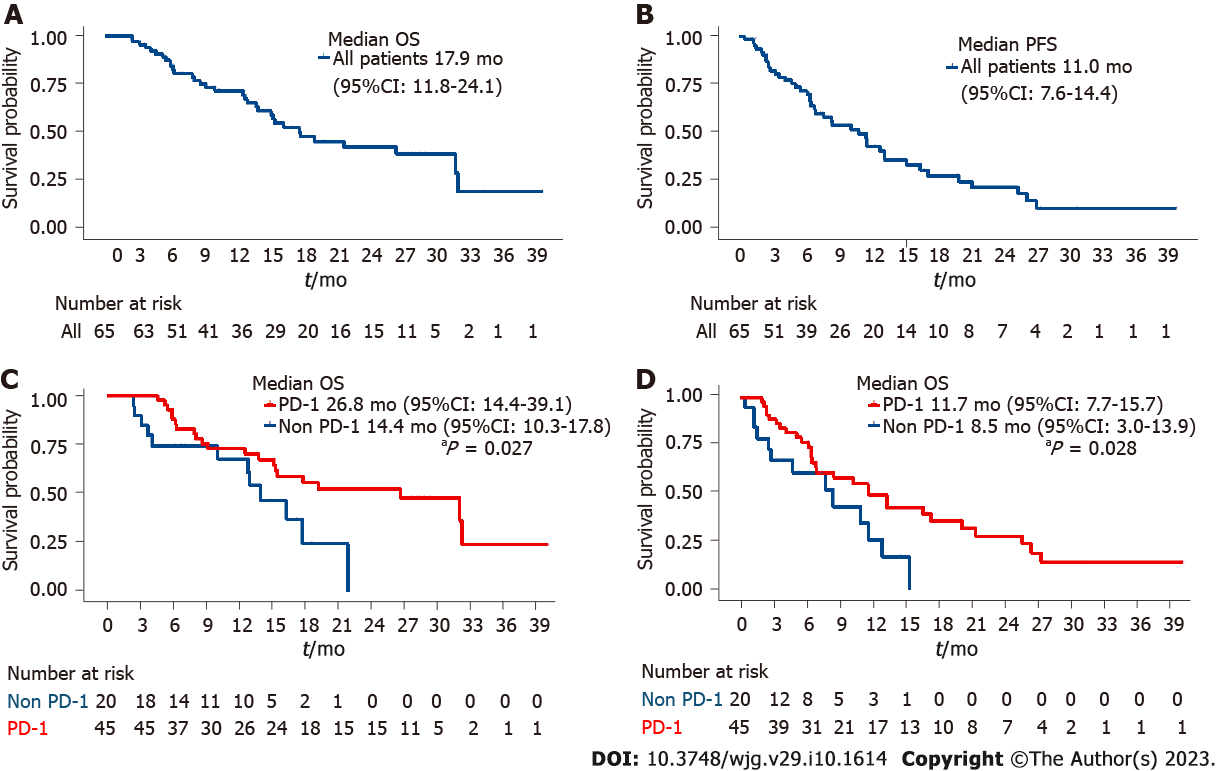
Figure 2 The overall survival and progression-free survival times of all included patients and programmed death receptor-1-lenvatinib-transarterial chemoembolization group and lenvatinib-transarterial chemoembolization group.
A: The overall survival (OS) times of all included patients; B: The progression-free survival (PFS) times of all included patients; C: The OS times of programmed death receptor-1 (PD-1)-lenvatinib-transarterial chemoembolization (TACE) group and lenvatinib-TACE group; D: The PFS times of PD-1-lenvatinib-TACE group and lenvatinib-TACE group. OS: Overall survival; PFS: Progression-free survival; CI: Confidence interval; PD-1: Programmed death receptor-1.
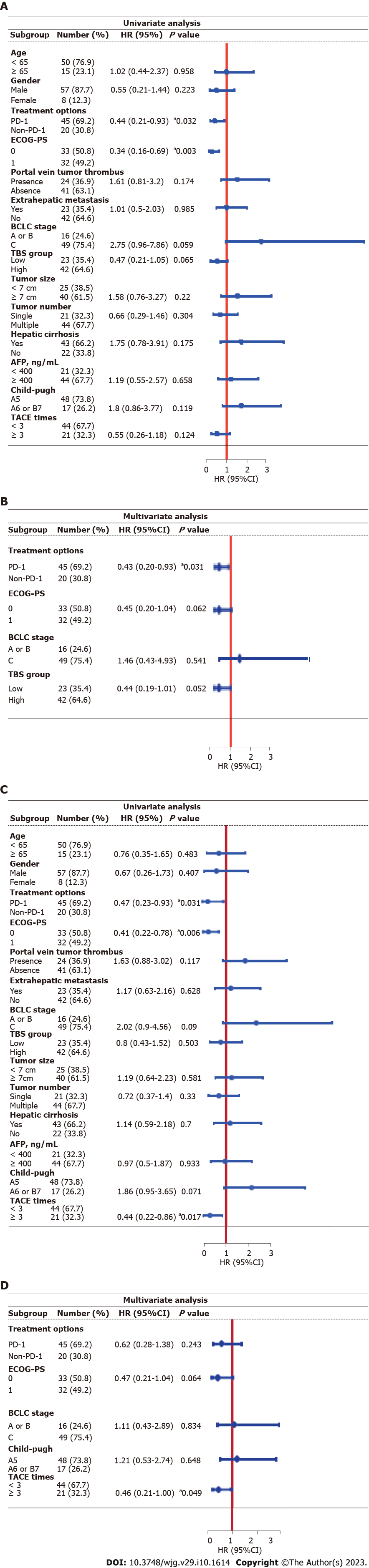
Figure 3 Univariate and multivariable Cox regression analysis for overall survival and progression-free survival.
A: Univariate Cox regression analysis for overall survival (OS); B: Multivariable Cox regression analysis for OS; C: Univariate Cox regression analysis for progression-free survival (PFS); D: Multivariable Cox regression analysis for PFS. aP < 0.05. HR: Hazard ratio; CI: Confidence interval; PD-1: Programmed death receptor-1; TACE: Transarterial chemoembolization; ECOG-PS: Eastern Cooperative Oncology Group performance status; BCLC: Barcelona Clinic Liver Cancer; AFP: Alpha-fetoprotein; TBS: Tumor burden score.
- Citation: Wang YY, Yang X, Wang YC, Long JY, Sun HS, Li YR, Xun ZY, Zhang N, Xue JN, Ning C, Zhang JW, Zhu CP, Zhang LH, Yang XB, Zhao HT. Clinical outcomes of lenvatinib plus transarterial chemoembolization with or without programmed death receptor-1 inhibitors in unresectable hepatocellular carcinoma. World J Gastroenterol 2023; 29(10): 1614-1626
- URL: https://www.wjgnet.com/1007-9327/full/v29/i10/1614.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i10.1614