Copyright
©The Author(s) 2022.
World J Gastroenterol. Jan 14, 2022; 28(2): 188-198
Published online Jan 14, 2022. doi: 10.3748/wjg.v28.i2.188
Published online Jan 14, 2022. doi: 10.3748/wjg.v28.i2.188
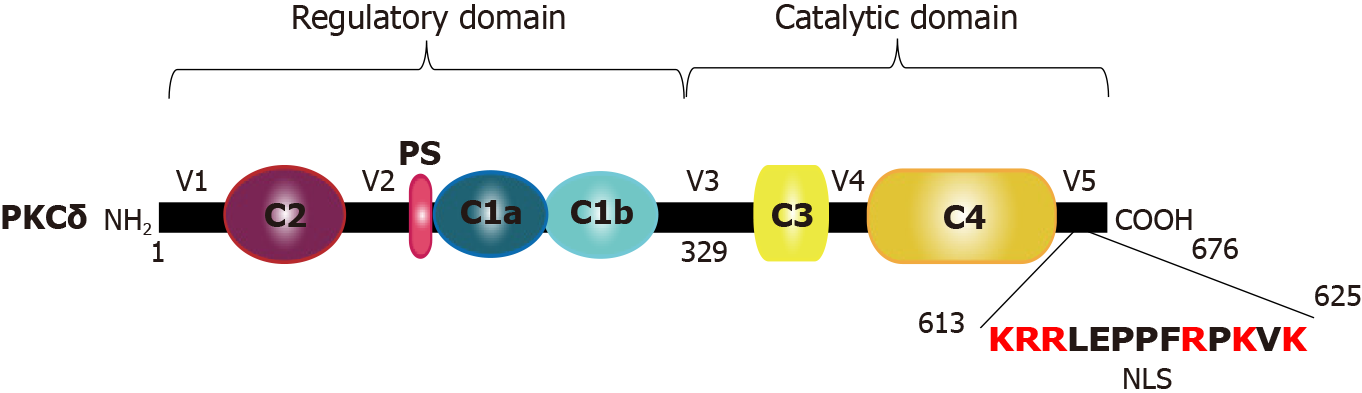
Figure 1 Schematic representation of protein kinase Cδ domains.
The N-terminal regulatory domain is composed of 1 to 329 amino acids, non-functional C2, pseudosubstrate, and lipid binding C1 (a and b) domains. The C-terminal catalytic domain is composed of 330 to 676 amino acids, ATP-binding C3, and kinase C4 domains. The 329 amino acids at the V3 region allow the cleavage site by caspase-3 to be constitutively active. The V5 region includes nuclear localization signal necessary for the nuclear transport of protein kinase Cδ. NLS: Nuclear localization signal.
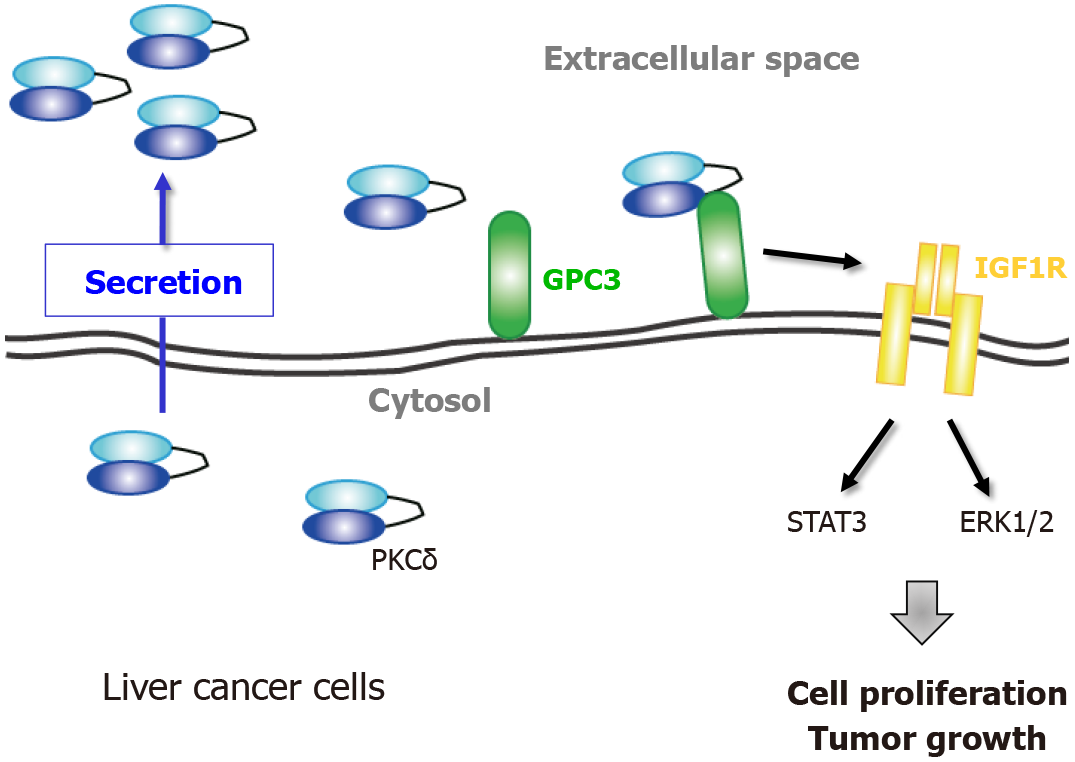
Figure 2 Extracellular protein kinase Cδ shows oncogenic property in liver cancer.
Model of the proliferative regulation of extracellular protein kinase Cδ (PKCδ) in liver cancer cells. PKCδ is secreted from living cells and resides at the plasma membrane through its association with glypican 3, leading to an increase in insulin-like growth factor 1 receptor activation and enhancement of subsequent proliferative signaling to increase cell growth. PKCδ: Protein kinase Cδ; GPC3: Glypican 3; ERK: Extracellular signal-regulated kinase; IGF1R: Insulin-like growth factor 1 receptor.
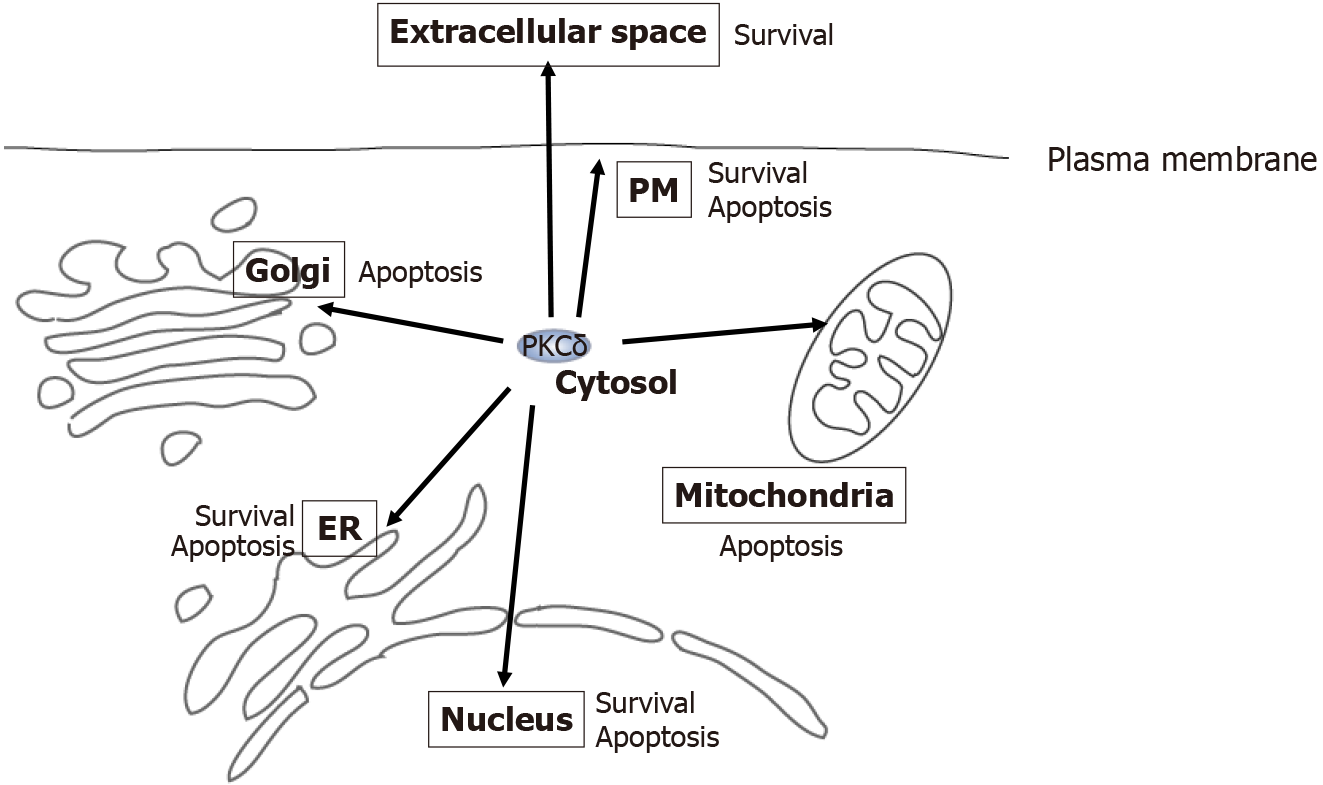
Figure 3 Multi-localization and functional diversity of protein kinase Cδ.
Protein kinase Cδ (PKCδ) resides at various locations, including the cytosol, nucleus, estrogen receptor (ER), mitochondria, Golgi, extracellular space, and plasma membrane (inside and outside the cell). At each location, PKCδ acts as an apoptotic or survival factor in response to various stimuli, such as genotoxic stresses, phorbol ester, DNA damage, ER stress, tumor necrosis factor (TNF)-α, and TNF-related apoptosis-inducing ligand. PKCδ: Protein kinase Cδ; ER: Estrogen receptor; PM: Plasma membrane.
- Citation: Yamada K, Yoshida K. Multiple subcellular localizations and functions of protein kinase Cδ in liver cancer. World J Gastroenterol 2022; 28(2): 188-198
- URL: https://www.wjgnet.com/1007-9327/full/v28/i2/188.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i2.188