Copyright
©The Author(s) 2021.
World J Gastroenterol. Jul 28, 2021; 27(28): 4493-4503
Published online Jul 28, 2021. doi: 10.3748/wjg.v27.i28.4493
Published online Jul 28, 2021. doi: 10.3748/wjg.v27.i28.4493
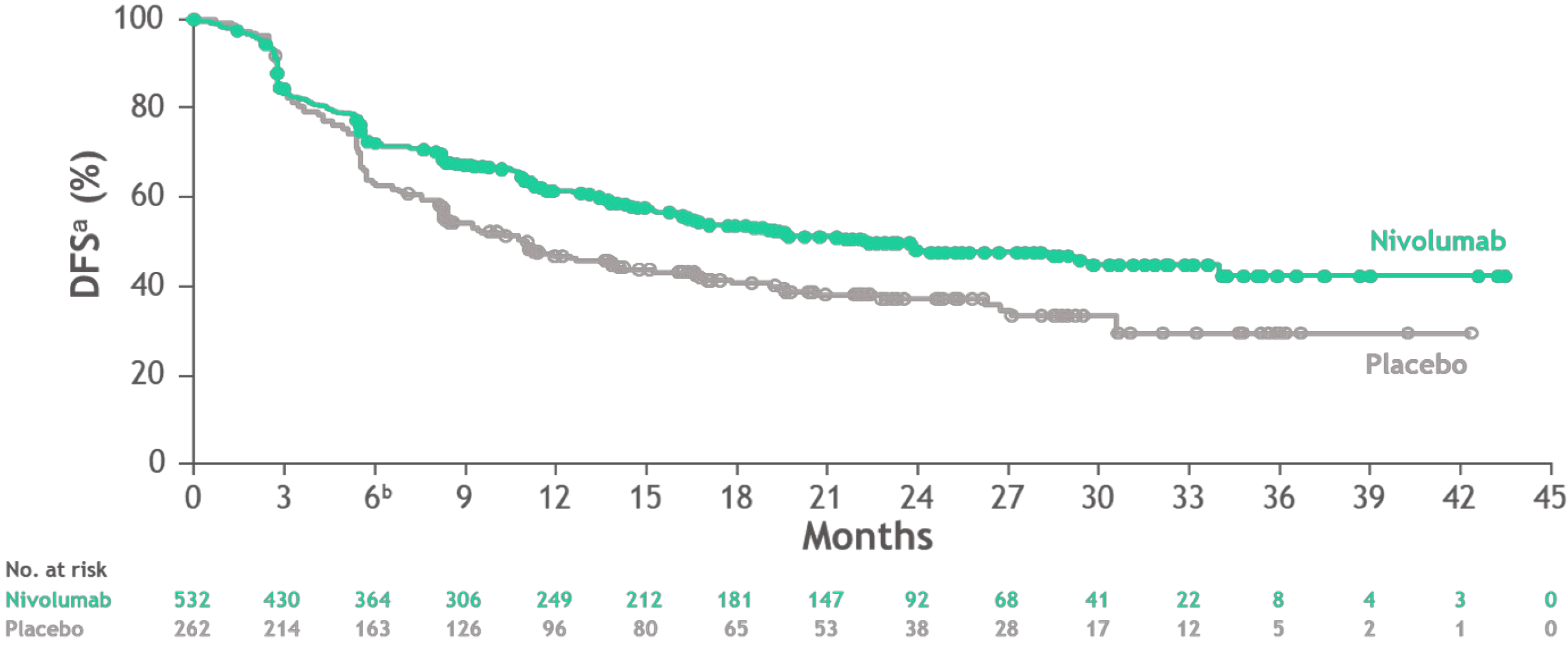
Figure 1 Disease-free survival in the study population (CheckMate 577).
Citation: Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, Mendez G, Feliciano J, Motoyama S, Lièvre A, Uronis H, Elimova E, Grootscholten C, Geboes K, Zafar S, Snow S, Ko AH, Feeney K, Schenker M, Kocon P, Zhang J, Zhu L, Lei M, Singh P, Kondo K, Cleary JM, Moehler M; CheckMate 577 Investigators. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N Engl J Med 2021; 384(13): 1191-1203. Copyright ©The Authors 2021. Published by Massachusetts Medical Society[7]. DFS: Disease-free survival.
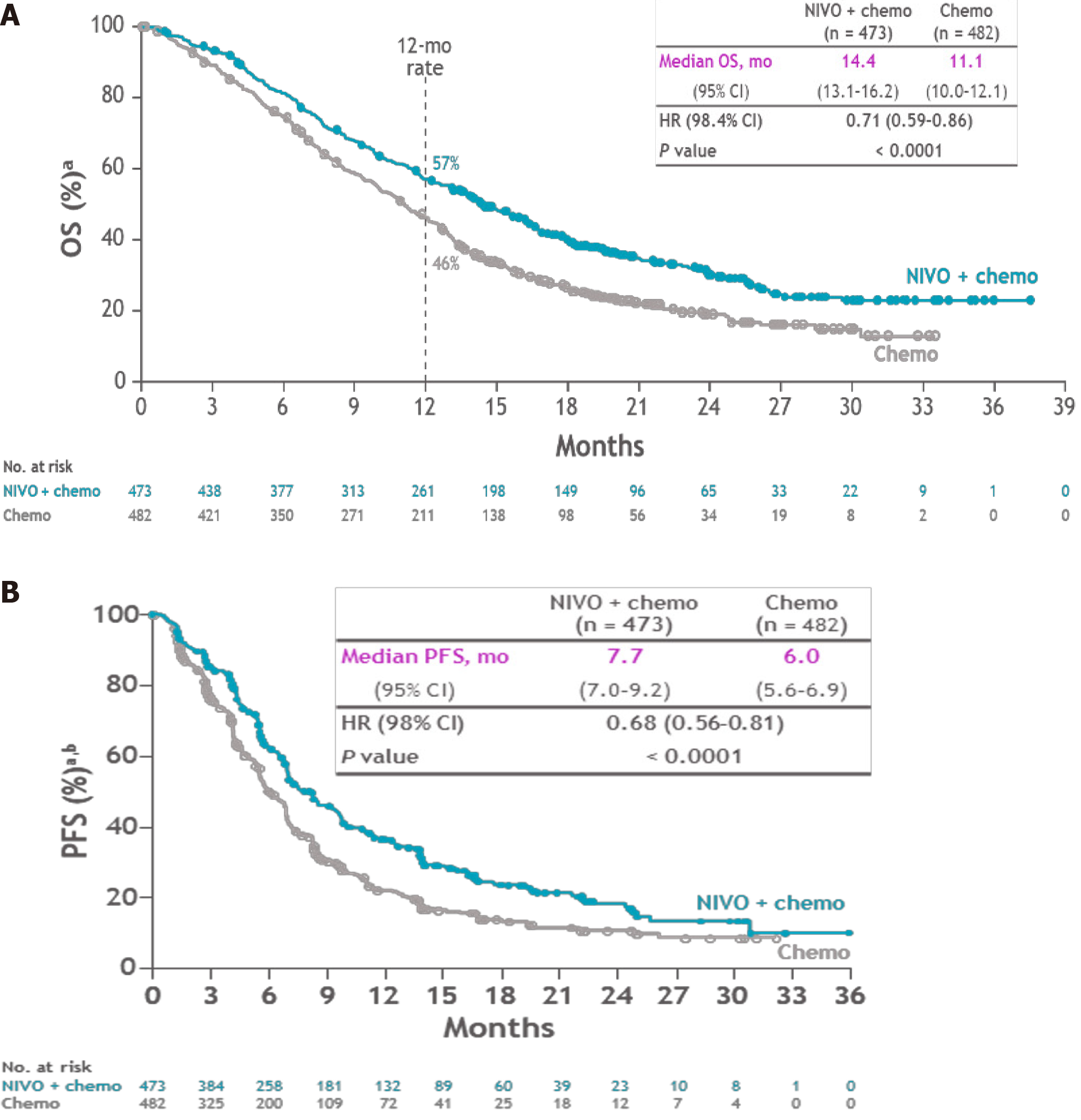
Figure 2 Overall survival and progression-free survival in patients with programmed cell death ligand 1 combined positive score of more than 5% (CheckMate 649).
A: Overall survival; B: Disease-free survival. Citation: Moehler M, Shitara K, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Bragagnoli AC, Liu T, Schenker M, Yanez P, Tehfe M, Poulart V, Cullen D, Lei M, Kondo K, Li M, Ajani JA, Janjigian YY. Nivolumab (nivo) plus chemotherapy (chemo) vs chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer (GC/GEJC)/esophageal adenocarcinoma (EAC): First results of the CheckMate 649 study. Ann Oncol 2020; 31(4): S1191. Copyright ©The Author(s). Published by European Society of Medical Oncology[12]. PFS: Progression-free survival; HR: Hazard ratio; CI: Confidence interval; OS: Overall survival.
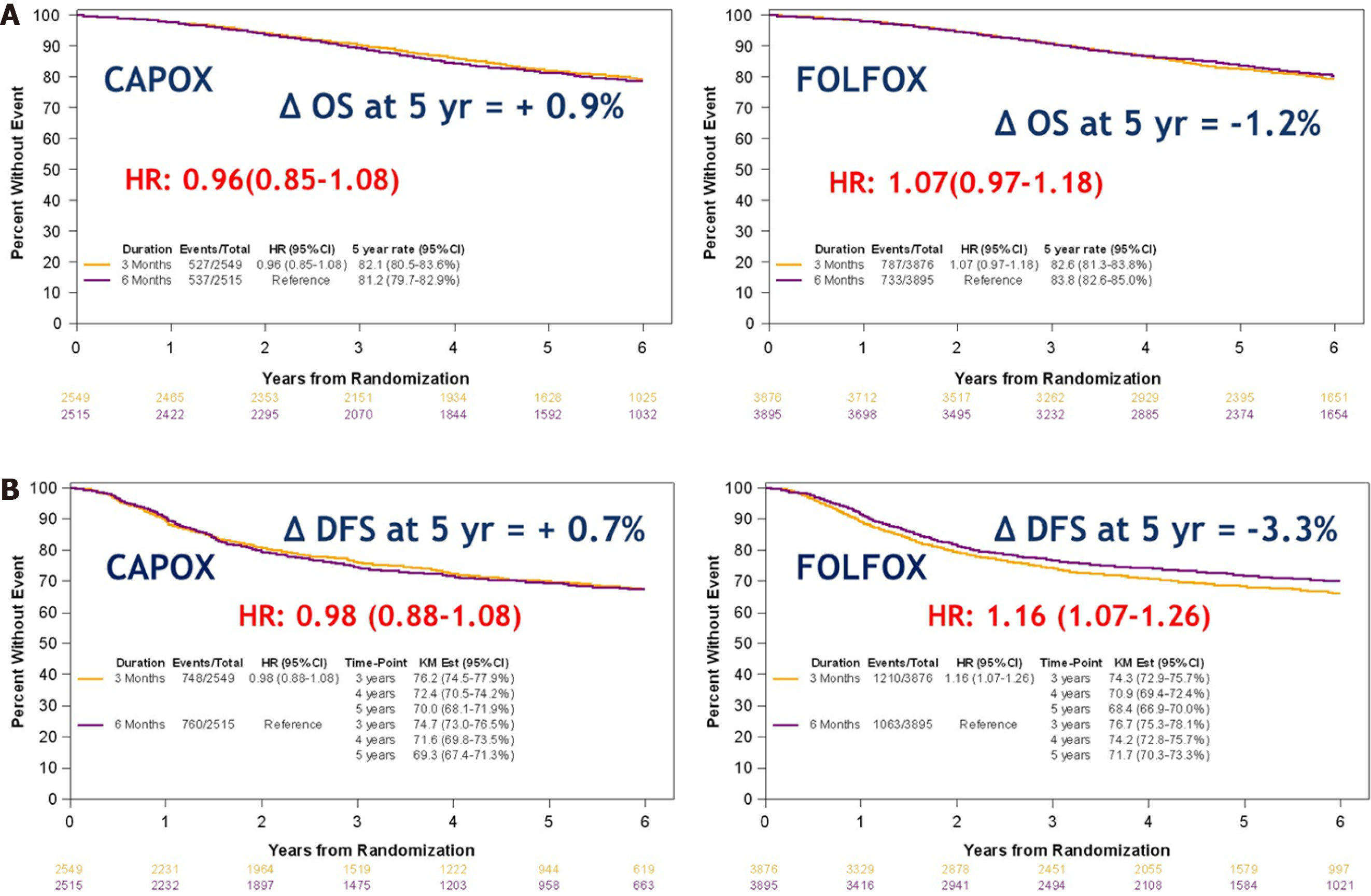
Figure 3 Overall survival and disease-free survival with 3 mo vs 6 mo of adjuvant therapy (international duration evaluation of adjuvant therapy).
A: Overall survival; B: Disease-free survival. Citation: Sobrero AF, Andre T, Meyerhardt JA, Grothey A, Iveson T, Yoshino T, Sougklakos I, Meyers JP, Labianca R, Saunders MP, Vernerey D, Yamanaka T, Boukovinas I, Oki E, Georgoulias V, Torri V, Harkin A, Taieb J, Shields AF, Shi Q. Overall survival (OS) and long-term disease-free survival (DFS) of three vs six months of adjuvant (adj) oxaliplatin and fluoropyrimidine-based therapy for patients (pts) with stage III colon cancer (CC): Final results from the IDEA (International Duration Evaluation of Adj chemotherapy) collaboration. J Clin Oncol 2020; 38(15): 4004-4004. Copyright ©The Author(s). Published by the American Society of Clinical Oncology[21]. DFS: Disease-free survival; HR: Hazard ratio; OS: Overall survival.
- Citation: Bordry N, Astaras C, Ongaro M, Goossens N, Frossard JL, Koessler T. Recent advances in gastrointestinal cancers. World J Gastroenterol 2021; 27(28): 4493-4503
- URL: https://www.wjgnet.com/1007-9327/full/v27/i28/4493.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i28.4493