Copyright
©The Author(s) 2020.
World J Gastroenterol. May 28, 2020; 26(20): 2498-2513
Published online May 28, 2020. doi: 10.3748/wjg.v26.i20.2498
Published online May 28, 2020. doi: 10.3748/wjg.v26.i20.2498
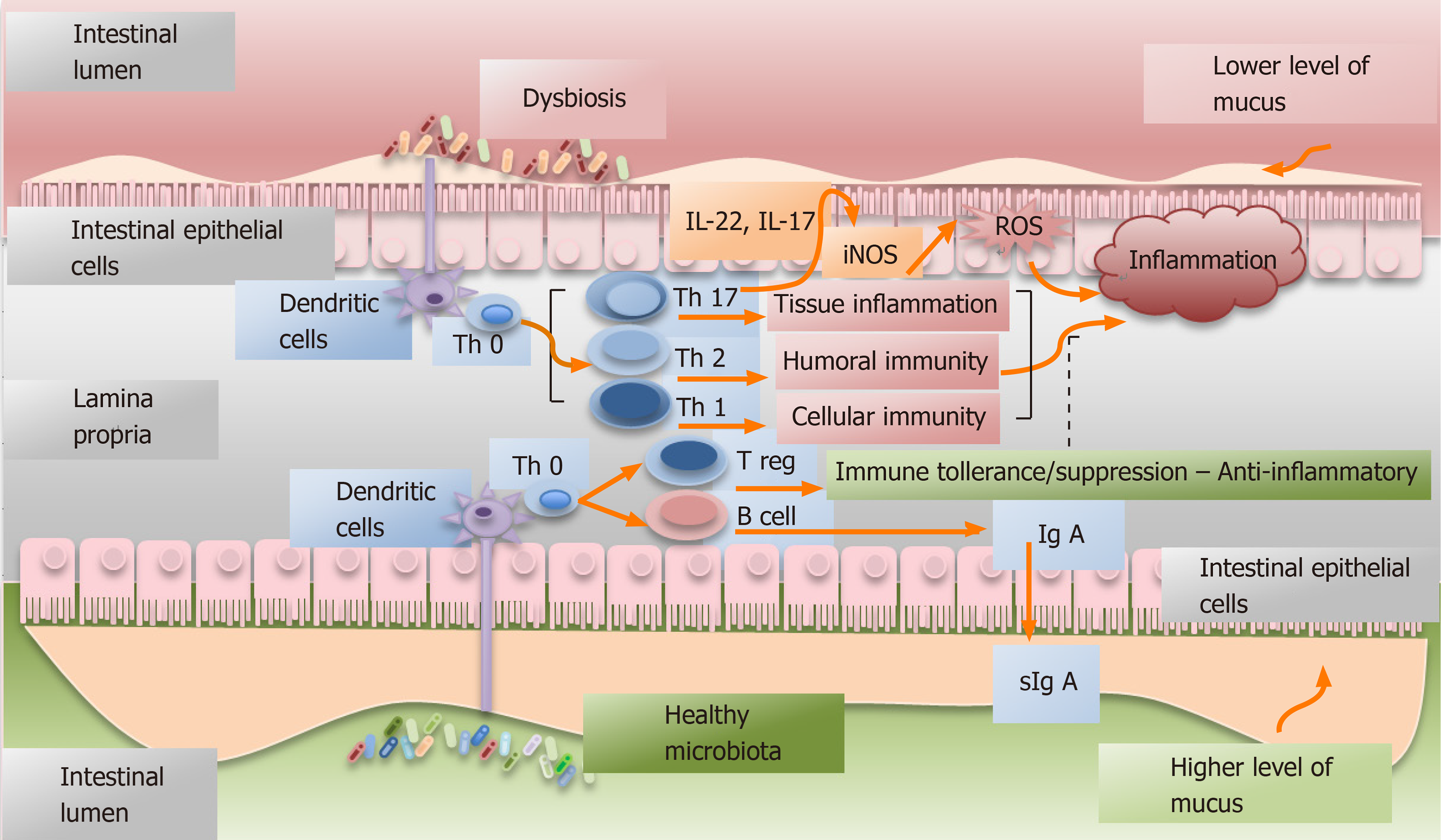
Figure 1 Simplified graphic depicting the interactions between the healthy or dysbiotic microbiota and immune system.
Dysbiosis is defined as the abnormal and predominant presence of pathogens in an environment or as alterations of the considered normal proportion of the different specimens composing the microbiota[1]. The microbiota largely contributes to the development of the lymphoid tissue[11] and it can modulate host immune system (innate and adaptive)[12]. Intestinal microbiota interacts with the immune response elements through dendritic cells. In dysbiosis, a Th17-type of immune response may be activated with consequent production of IL-17 and IL-22, both having a pro-inflammatory and pro-tumoral effect[2]. Furthermore, IL-22 can favor the expression of inducible nitric oxide synthase and the subsequently production of oxygen reactive species that are linked to cancer promotion[45].
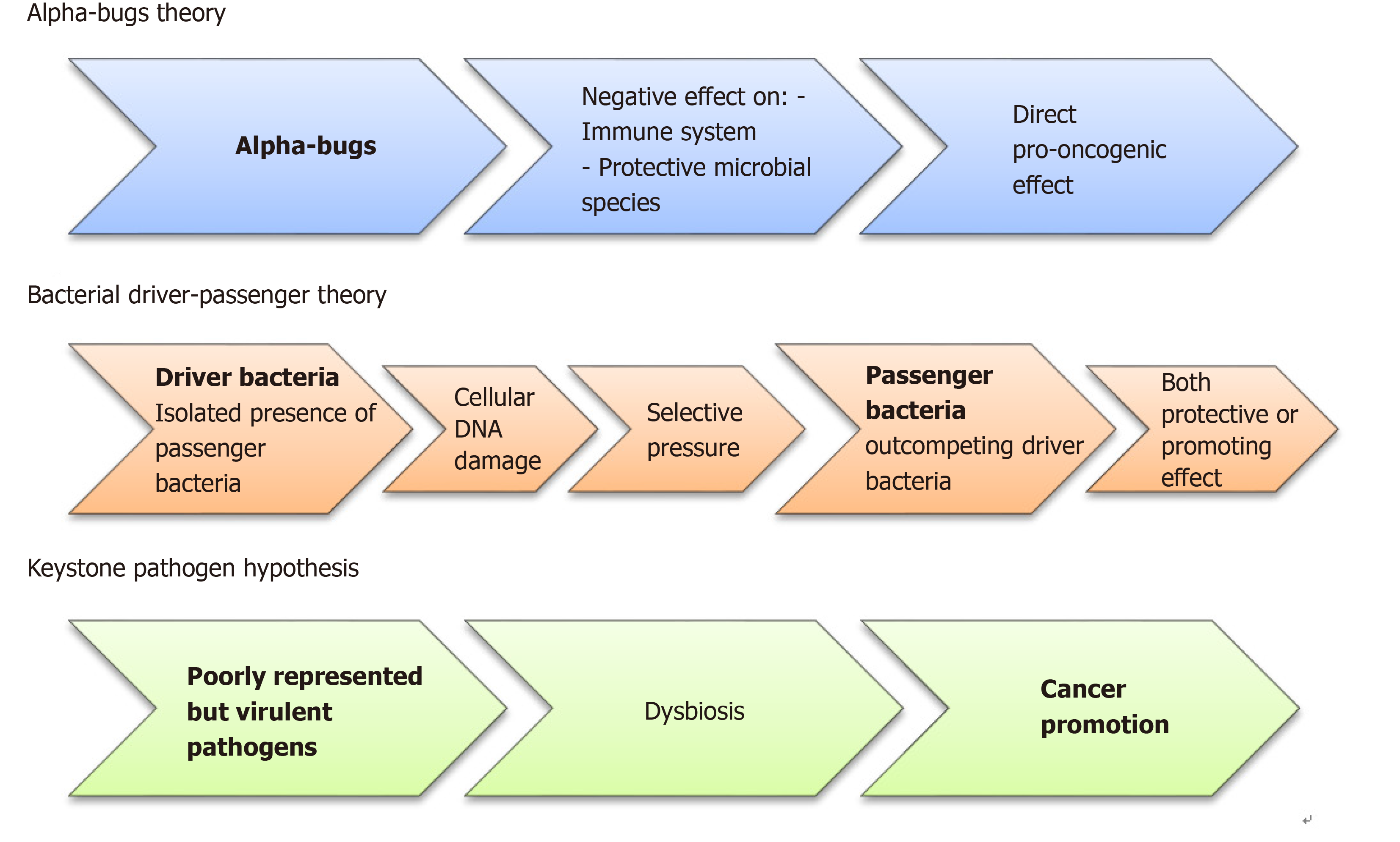
Figure 2 Diagram showing the three pathogenetic models involved in colorectal cancer initiation and promotion.
Currently, to explain the colorectal cancer development, three different pathogenetic models have been suggested. According to the “alfa-bugs” model, some species (e.g., Bacteroides fragilis) may have direct pro-oncogenic effect acting against both immune system and protective microbial species[22]. The “bacterial driver-passenger” model suggests that some “driver bacteria” promote cancer development through DNA damage. Subsequently, the “passenger bacteria” replace them having protective or cancer-promoting activities. The results of this new balance will determine tumor progression or tissue healing[23]. Finally, in the “keystone pathogen” model, some poorly represented pathogens have the unbalanced ability to alter the equilibrium within the normal microbiota causing a dysbiosis[25].
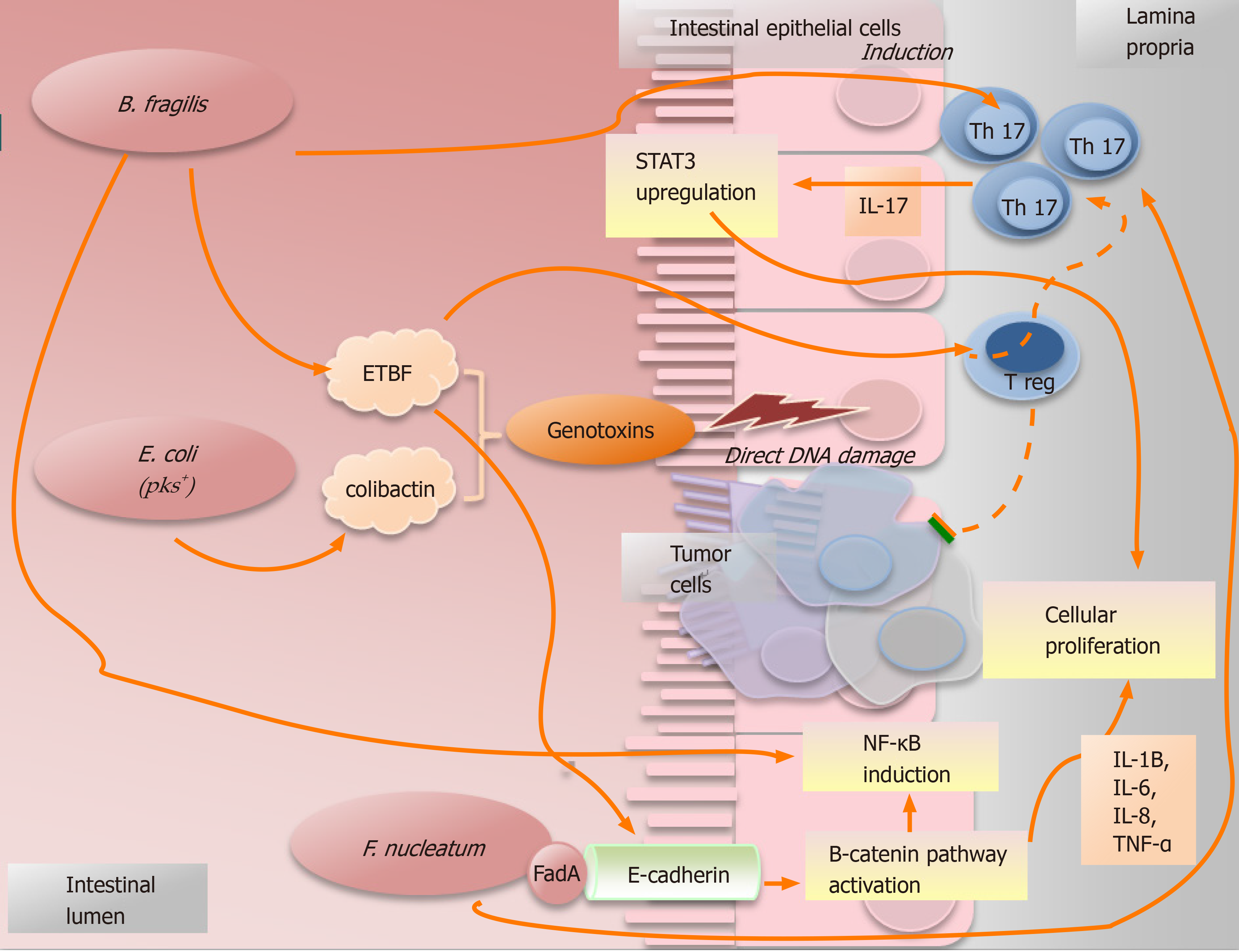
Figure 3 Simplified picture illustrating some examples of how microorganisms promote the cancer.
Bacteroides fragilis can cause the induction of Th17-type immune response with upregulation of signal transducer and transcription-3. Moreover, some subtypes of Bacteroides fragilis can secrete the toxin EBTF that can cause cancer in different ways: (1) Through direct DNA damage; (2) By Treg cells, which in presence of EBTF, seem to promote cancer progression through Th17 expansion[43]; and (3) Through the stimulation of the cleavage of E-cadherin which causes cellular proliferation and intestinal barrier breakage[23]. Nonetheless, B. fragilis can promote an inflammatory response inducing the signaling NF-κB pathway[44]. The particular group of polyketide synthase (pks) positive Escherichia coli (E. coli pks+) maintained a genomic island called “pks”. These bacteria can produce the genotoxin “colibactin”, that is able to induce direct DNA damage and, consequently, to increase the frequency of gene mutations[24,39]. Fusobacterium nucleatum can activate the β-catenin signal pathway causing cellular proliferation as consequence of the bindings between the bacterial adhesin FadA and E-cadherin which is located on the cells of the intestinal epithelium[31]. Furthermore, it is related to an increased production of some pro-cancer cytokines, such as TNF-α, IL-6, IL-12 and IL-17. STAT3: Signal transducer and transcription-3; ETBF: Enterotoxigenic Bacteroides fragilis; E. coli: Escherichia coli; F. nucleatum: Fusobacterium nucleatum.
- Citation: Bartolini I, Risaliti M, Ringressi MN, Melli F, Nannini G, Amedei A, Muiesan P, Taddei A. Role of gut microbiota-immunity axis in patients undergoing surgery for colorectal cancer: Focus on short and long-term outcomes. World J Gastroenterol 2020; 26(20): 2498-2513
- URL: https://www.wjgnet.com/1007-9327/full/v26/i20/2498.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i20.2498