Copyright
©The Author(s) 2020.
World J Gastroenterol. Jan 14, 2020; 26(2): 109-133
Published online Jan 14, 2020. doi: 10.3748/wjg.v26.i2.109
Published online Jan 14, 2020. doi: 10.3748/wjg.v26.i2.109
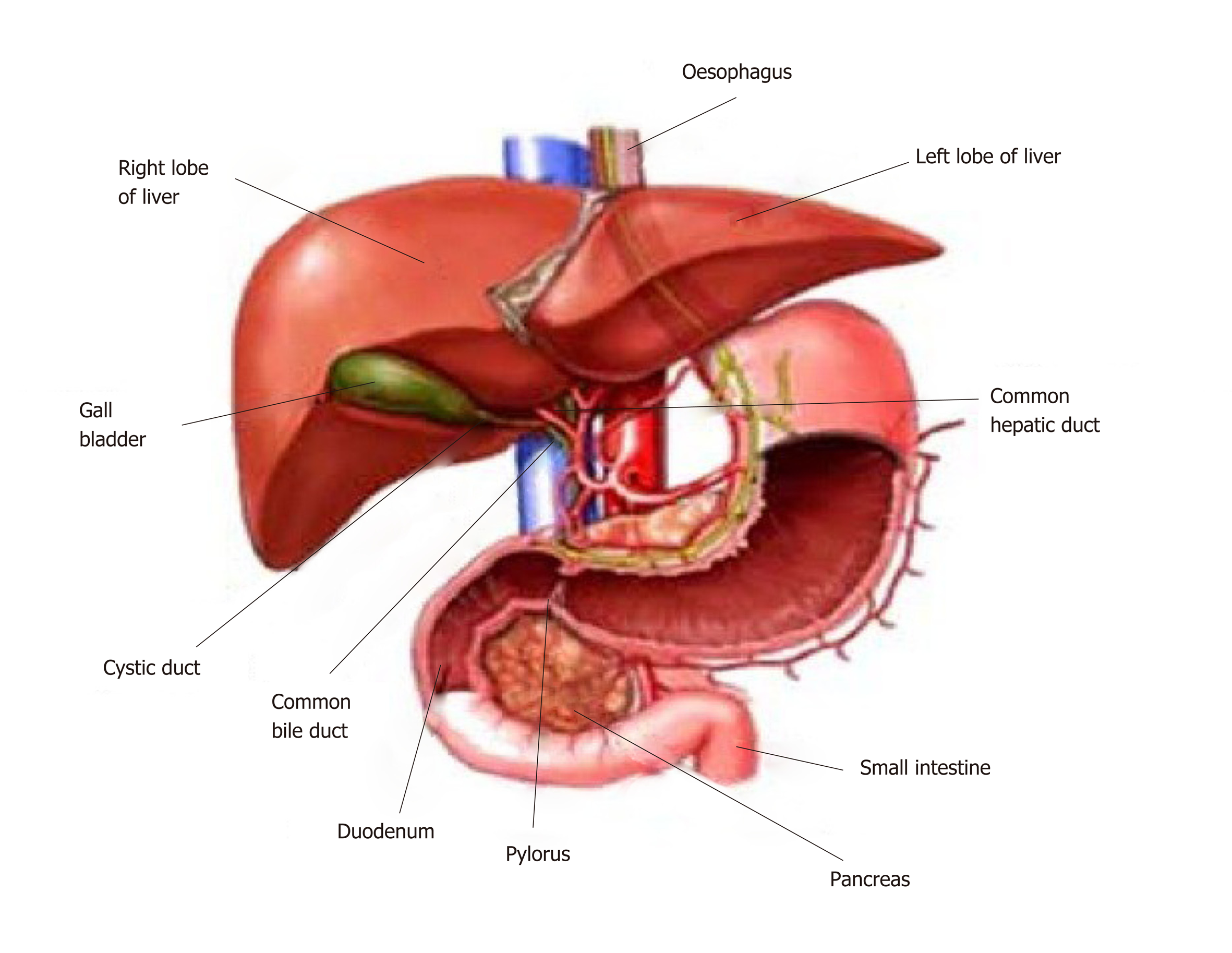
Figure 1 Anatomy of the liver and its macroscopic relationship to the intestinal tract and vasculature.
Reproduced with permission of Creative Commons Attribution License from Ebaid et al[164].
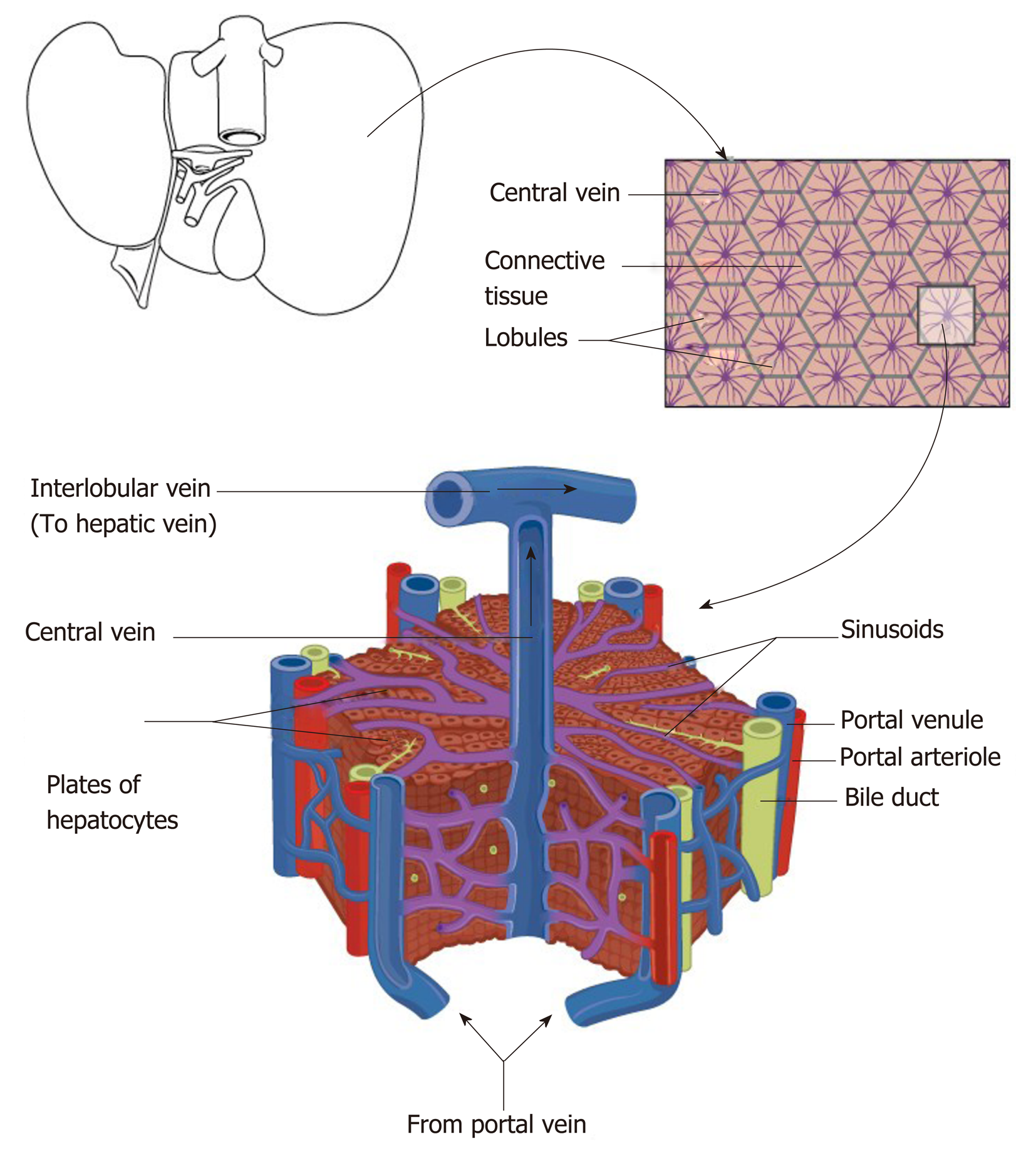
Figure 2 Schematic diagram representing the relationship of the macroscopic structure of the liver with the functional hepatic lobule with hepatic venules (blue), hepatic arteriole (red), bile ductules (yellow).
Reproduced with permission of Creative Commons Attribution License from Anatomy & Physiology textbook[165].
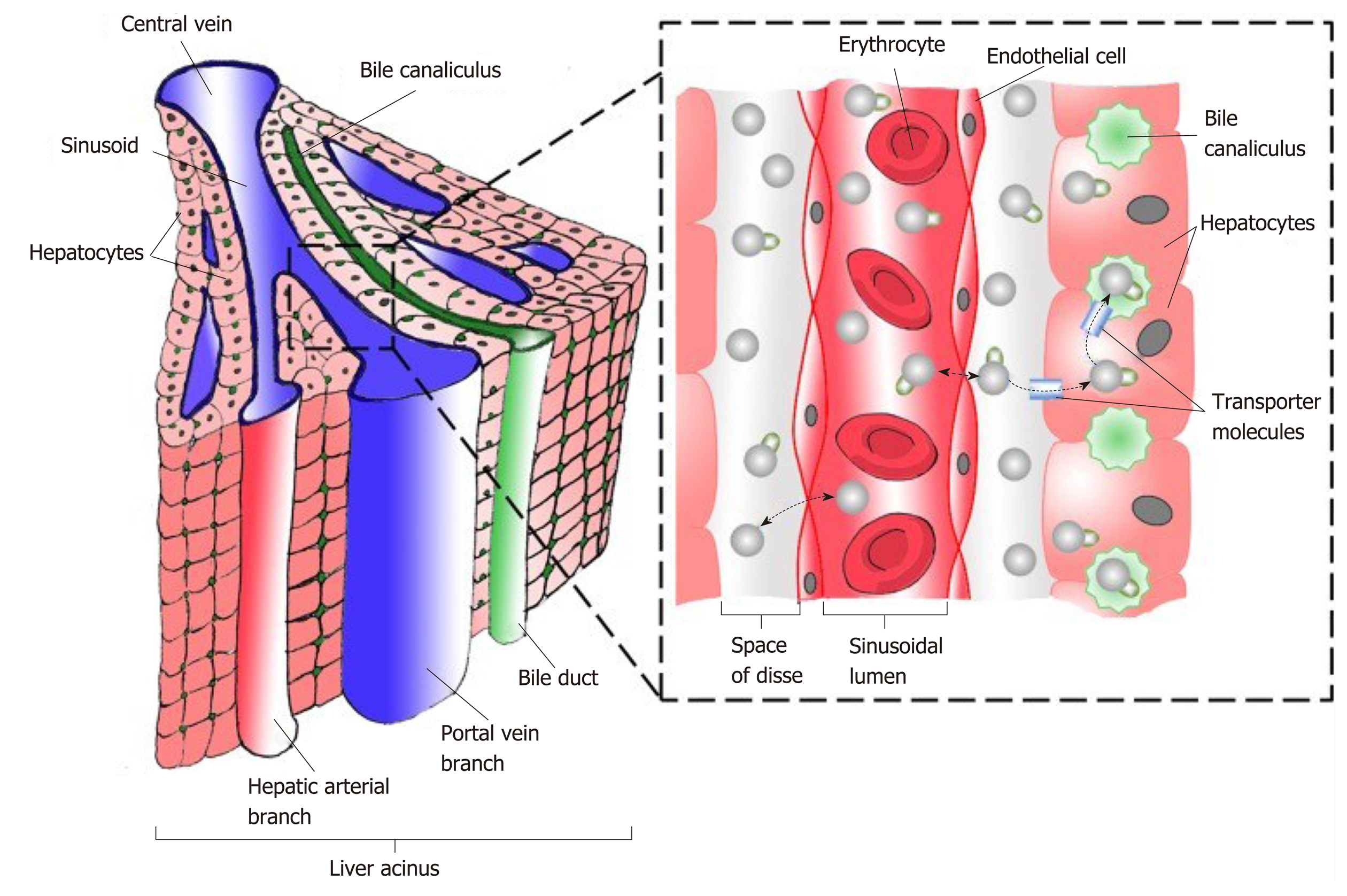
Figure 3 Schematic diagram representing functional hepatic acinus with hepatic venules (blue), hepatic arteriole (red), bile ductules (green) together with the relationship to the Space of Disse and the sinusoidal lumen.
Reproduced with permission of Creative Commons Attribution License from Chouhan et al[166].
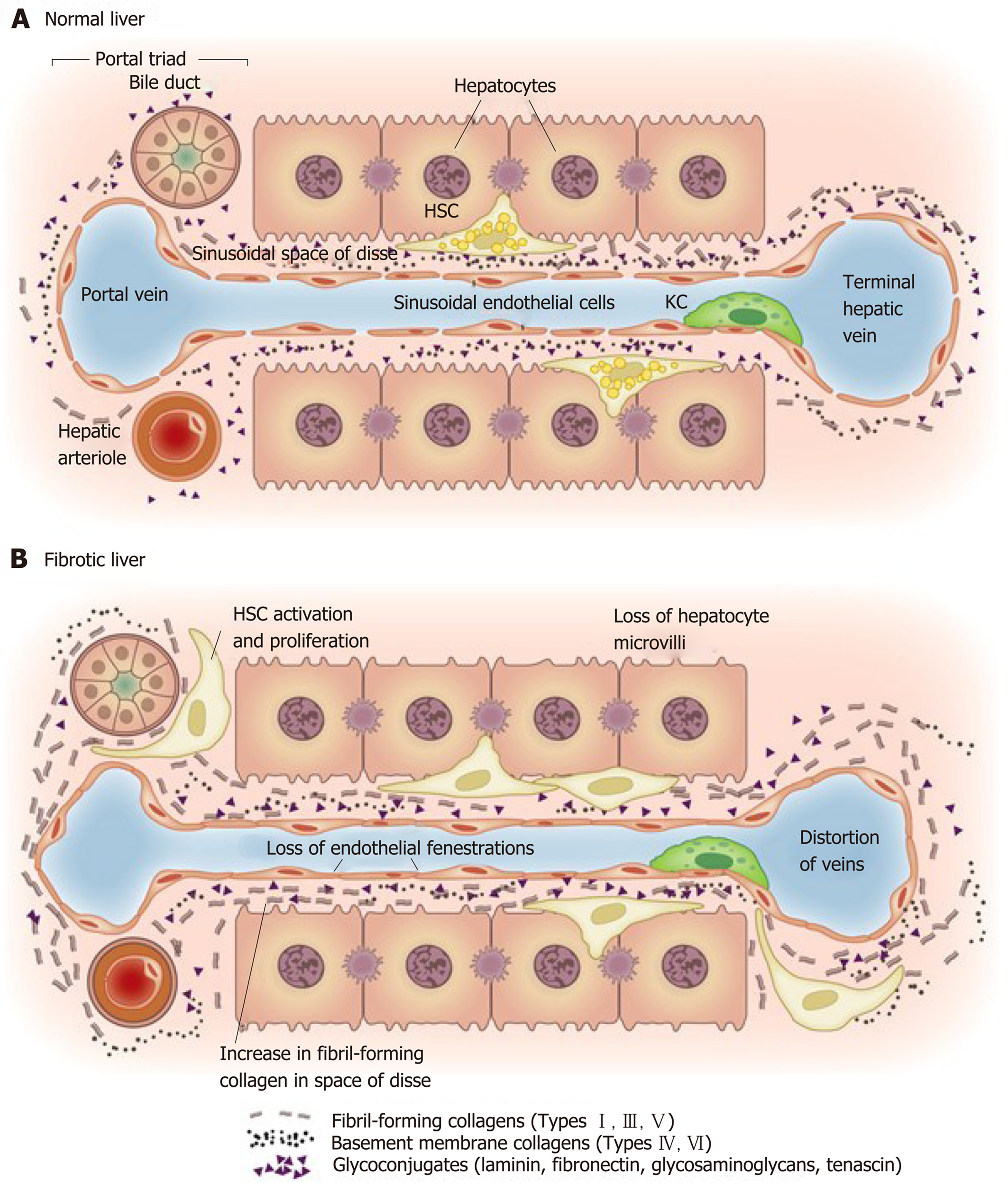
Figure 4 Matrix and cellular alteration in hepatic fibrosis.
Normal liver parenchyma contains epithelial cells (hepatocytes) and nonparenchymal cells: fenestrated sinusoidal endothelium, hepatic stellate cells, and Kupffer cells. A: After injury, the stellate cells become activated and secrete large amounts of extracellular matrix (ECM); B: Deposition of ECM in the space of Disse leads to the loss of both endothelial fenestrations and hepatocyte microvilli. Reproduced with permission from Hernandez-Gea and Friedman[37]. HSC: Hepatic stellate cells; KC: Kupffer cells.
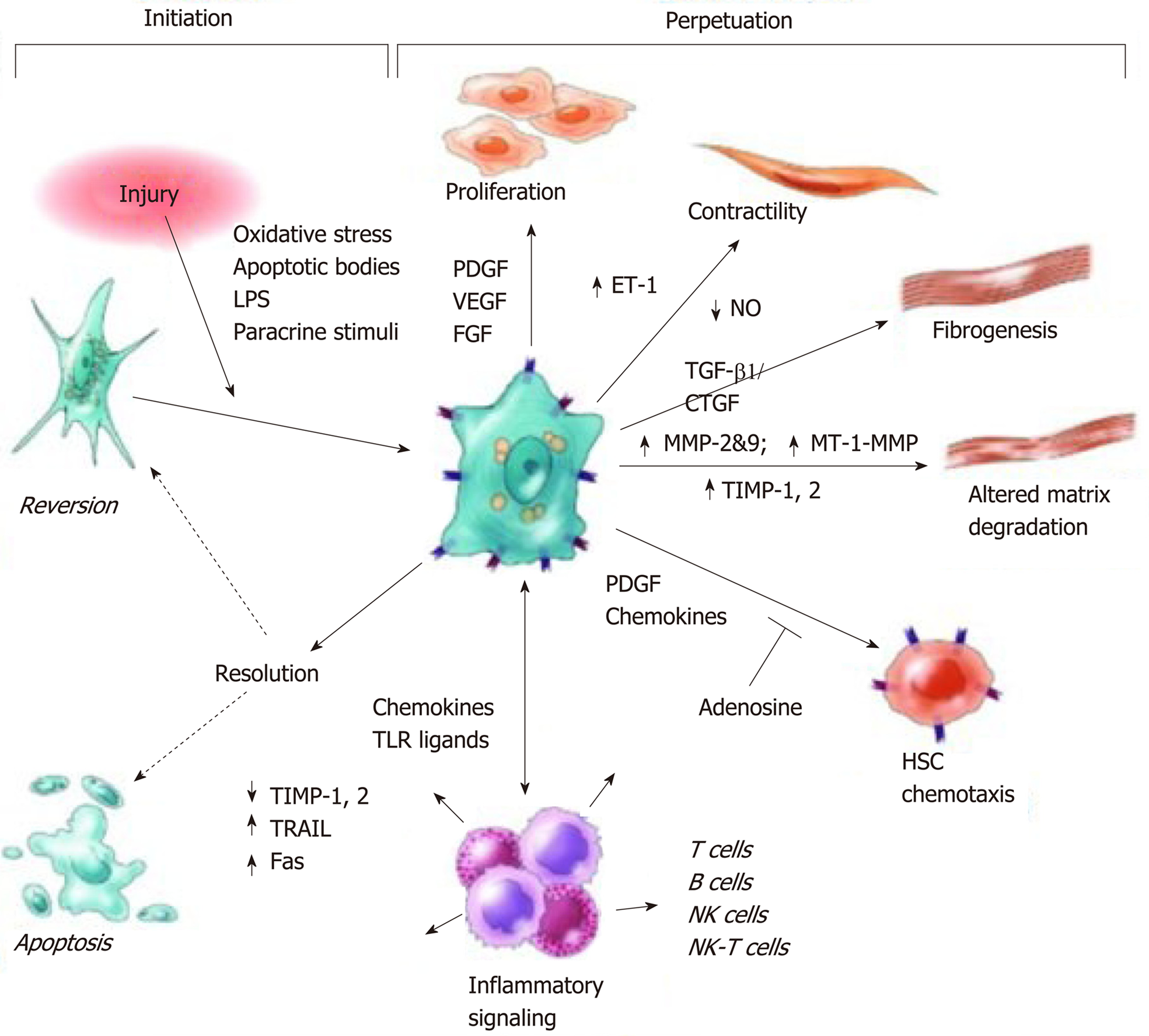
Figure 5 Pathways of hepatic stellate cell activation including those contributing to initiation and perpetuation.
Initiation is provoked by soluble stimuli that include oxidant stress signals, apoptotic bodies, lipopolysaccharide, and cytokine stimuli from neighbouring cells. Perpetuation is characterised by specific phenotypic changes including proliferation, contractility, fibrogenesis, altered matrix degradation, chemotaxis, and cytokine signalling. Reproduced with permission from Friedman[30]. CTGF: Connective tissue growth factor; ET: Endothelin; FGF: Fibroblast growth factor; LPS: Lipopolysaccharide; MMP: Matrix metalloproteinase; MT-1: membrane type-1, NK: Natural killer, NO: Nitrous oxide; PDGF: Platelet derived growth factor; TIMP: Tissue inhibitor of metalloproteases; TGF: Transforming growth factor; TLR: Toll like receptor; TRAIL: Tumour necrosis factor-related apoptosis-inducing ligand; VEGF: Vascular endothelial growth factor.
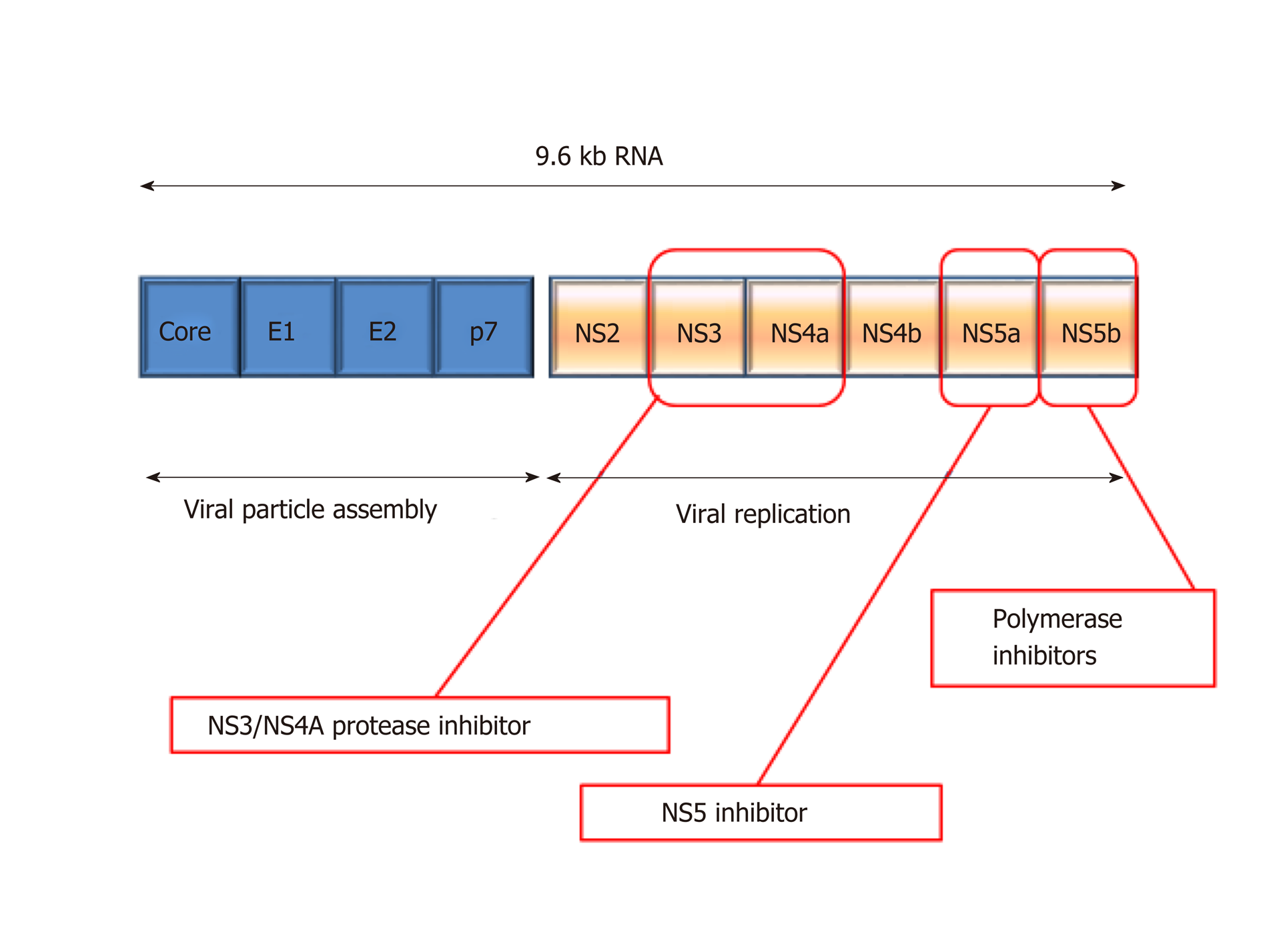
Figure 6 Depiction of hepatitis C virus genome structure and drug targets.
NS: Non-structural.
- Citation: Tanwar S, Rhodes F, Srivastava A, Trembling PM, Rosenberg WM. Inflammation and fibrosis in chronic liver diseases including non-alcoholic fatty liver disease and hepatitis C. World J Gastroenterol 2020; 26(2): 109-133
- URL: https://www.wjgnet.com/1007-9327/full/v26/i2/109.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i2.109