Copyright
©The Author(s) 2019.
World J Gastroenterol. May 14, 2019; 25(18): 2188-2203
Published online May 14, 2019. doi: 10.3748/wjg.v25.i18.2188
Published online May 14, 2019. doi: 10.3748/wjg.v25.i18.2188
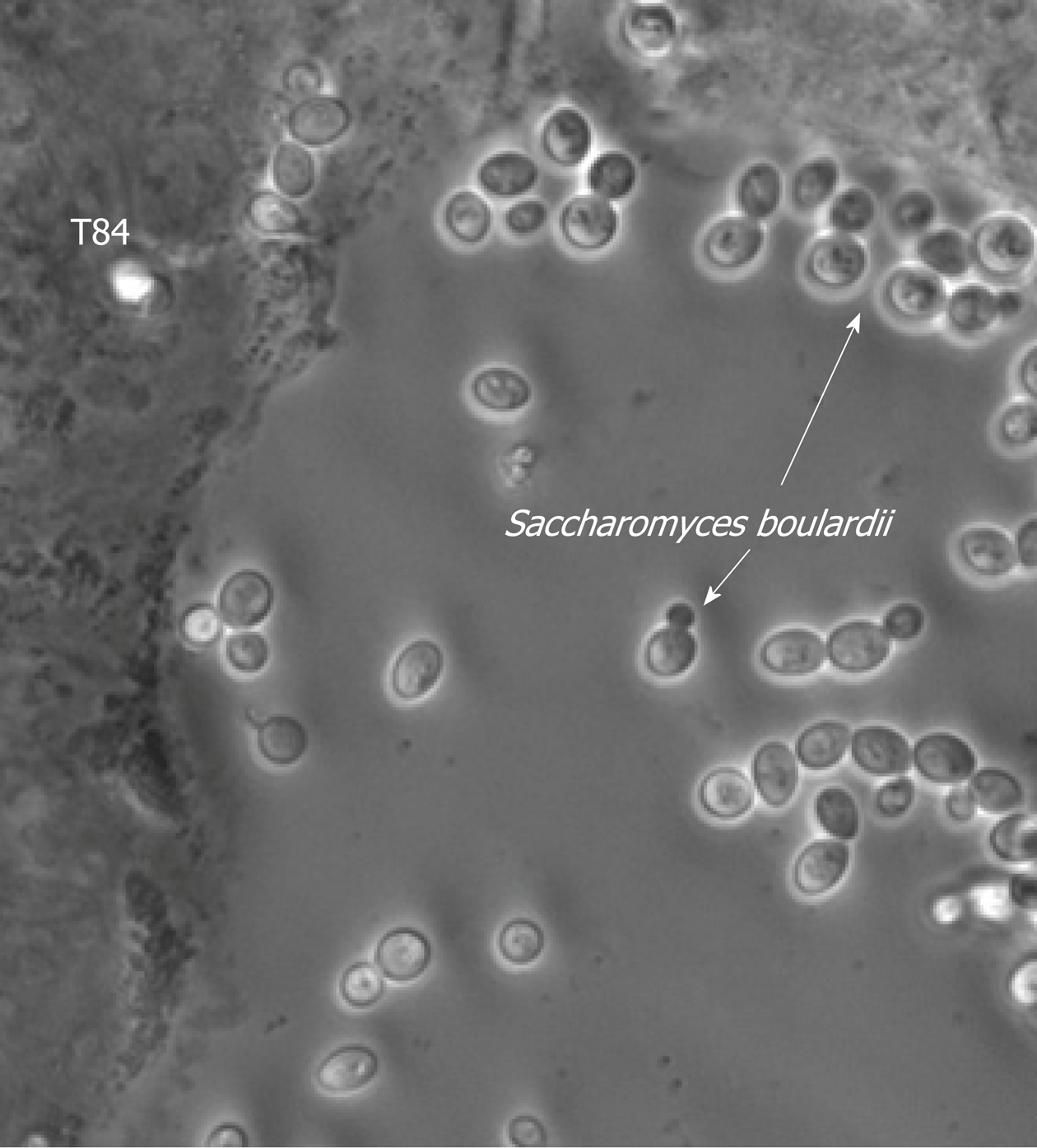
Figure 1 Transmission microscopy image showing Saccharomyces boulardii CNCM I-745 on a culture of human epithelial cells (T84 lineages).
(Source: Pontier-Bres R and Czerucka D). The arrows indicate budding yeast located either in the spaces between the cells or near the cell walls and the formation of a protective barrier.
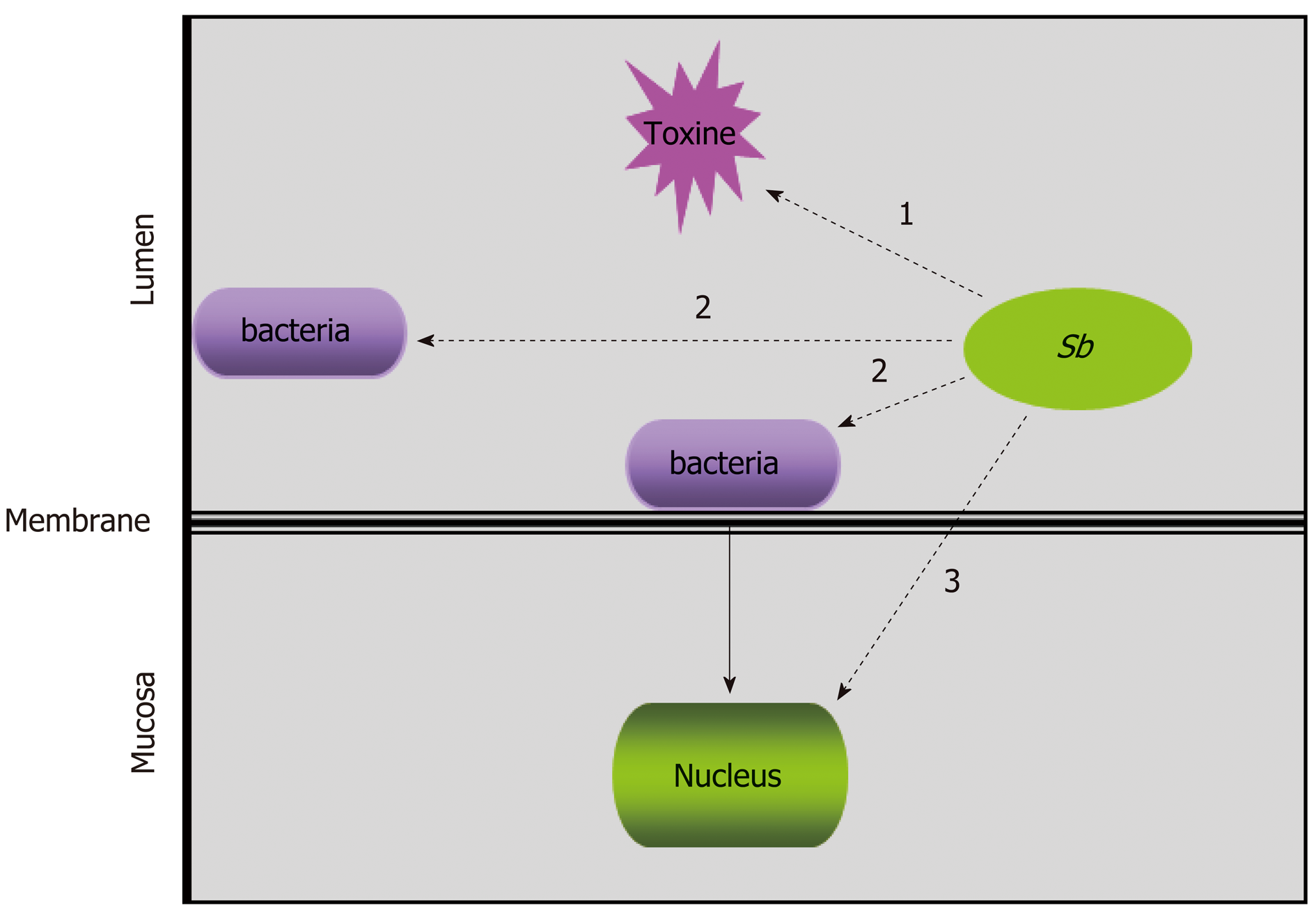
Figure 2 The hypothesized targets of Saccharomyces boulardii CNCM I-745 during bacterial infections: Saccharomyces boulardii CNCM I-745 may act directly on toxins “1”, on pathogenic bacteria “2” or on host cells “3”.
Sb: Saccharomyces boulardii CNCM I-745.
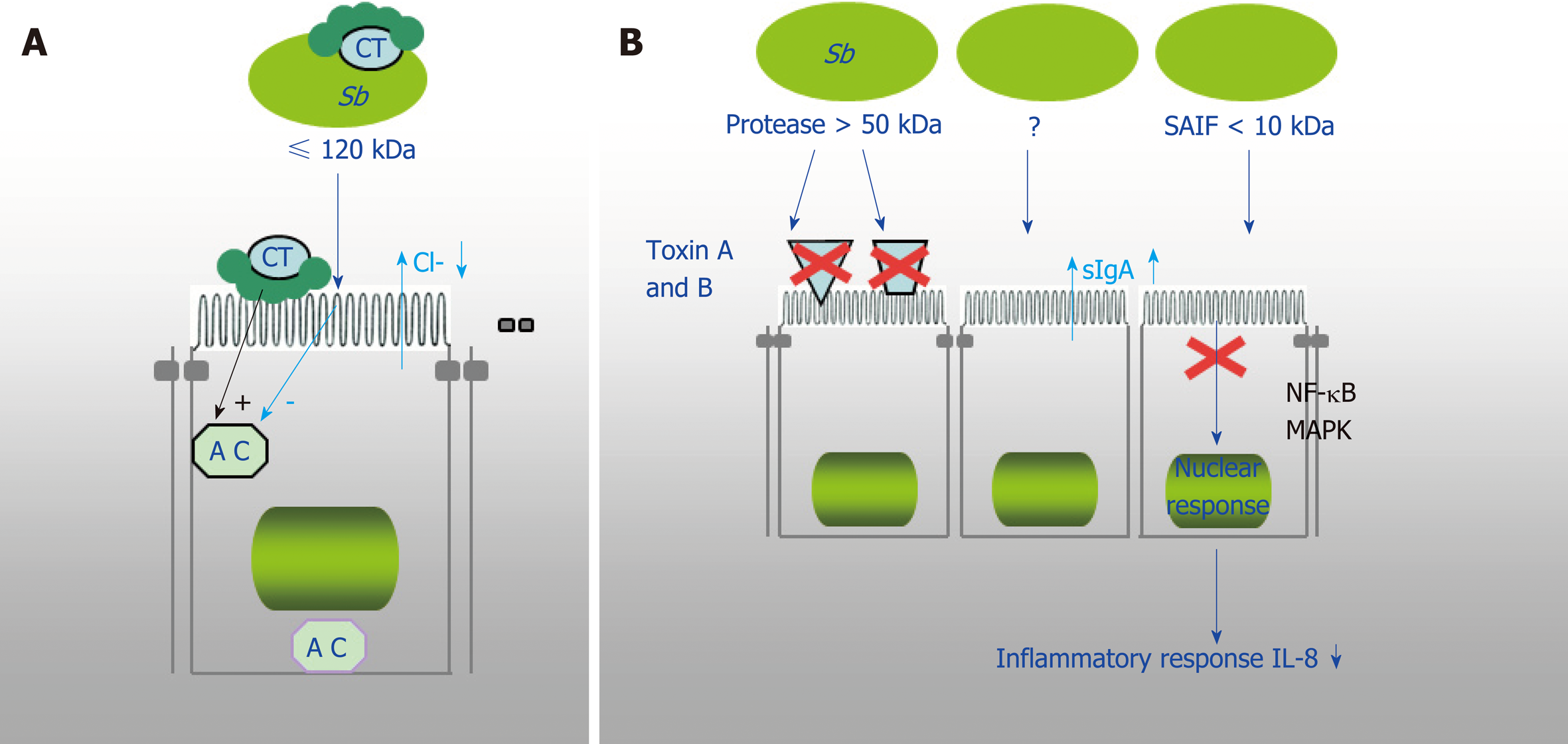
Figure 3 Demonstrated mechanism of Saccharomyces boulardii CNCM I-745 action against bacterial toxins.
A: Saccharomyces boulardii CNCM I-745 (S. b) produces a 120 kDa protein that inhibits adenylate cyclase and cholera toxin (CT)-induced chloride secretion. S. boulardii CNCM I-745 can also bind to CT; B: S. boulardii CNCM I-745 secretes a protease (> 54kDa) that lyses C. difficile toxins A and B and their receptors and a protein (< 10 kDa) that inhibits the signaling pathways involved in interleukin 8 synthesis. AC: Adenylate cyclase; CT: Cholera toxin; Sb: Saccharomyces boulardii CNCM I-745; IL-8: Interleukin 8.
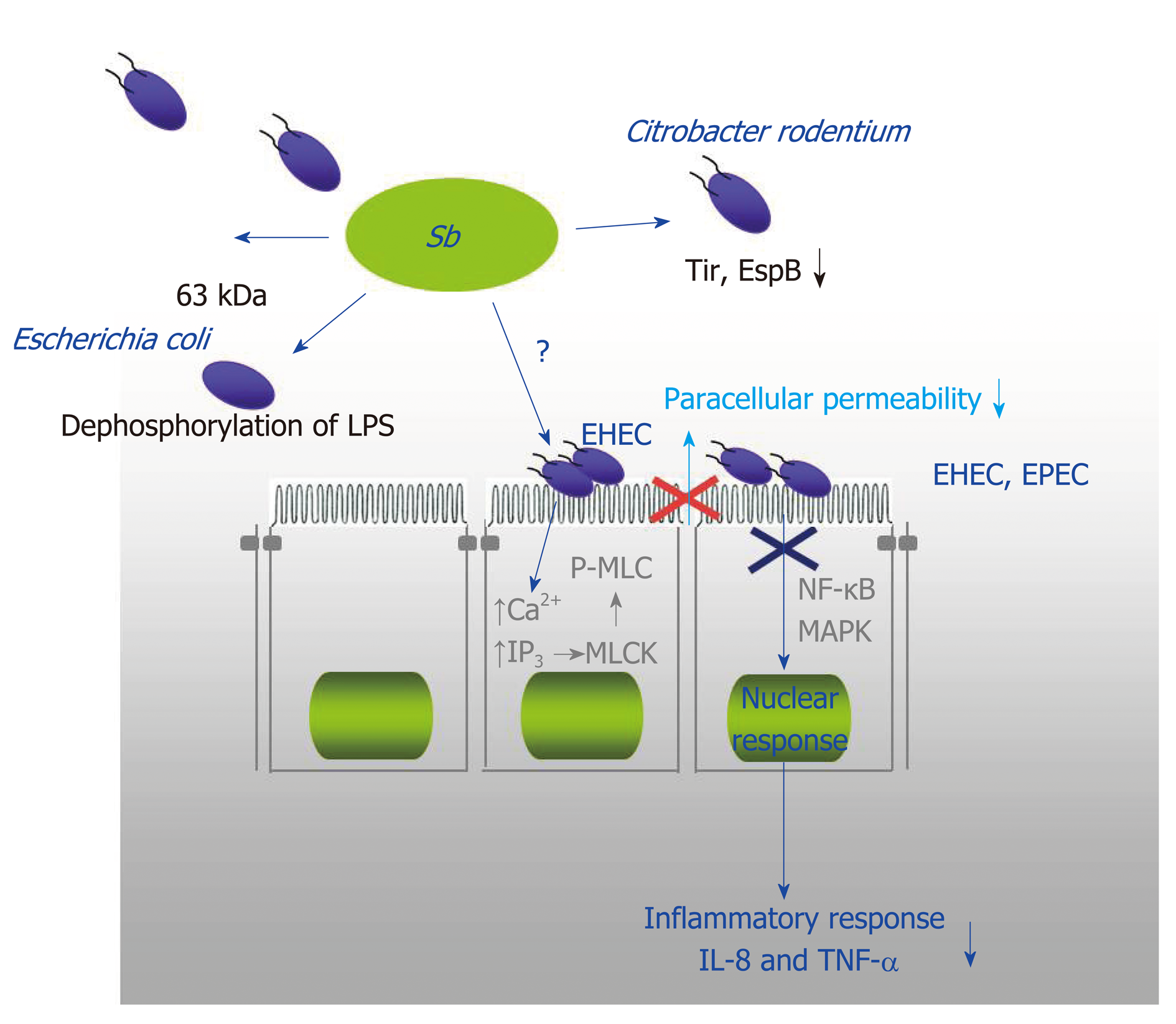
Figure 4 The protective mechanisms against enteropathogenic Escherichia coli (enteropathogenic coli, enterohaemorrhagic coli and Citrobacter rodentium).
They include an effect on the mucosa with Saccharomyces boulardii CNCM I-745 inhibiting the pathways involved in opening tight junctions (phosphorylation of myosin light chain kinase), inhibition of activation pathways of mitogen-activated protein kinases and NF-κB that are involved in the synthesis of Interleukin 8, and finally a direct effect of S. boulardii CNCM I-745 on bacteria (dephosphorylation of lipopolysaccharide of Escherichia coli and modification of the expression of pathogenicity factors of Citrobacter rodentium). EHEC: Enterohaemorrhagic coli; EPEC: Enteropathogenic coli; LPS: Lipopolysaccharide; MAPK: Mitogen-activated protein kinase; MLCK: Myosin light chain kinase; P-MLC: Phosphorylation of myosin light chain; IL-8: Interleukin 8; TNF-α: Tumor necrosis factor α Sb: Saccharomyces boulardii CNCM I-745.
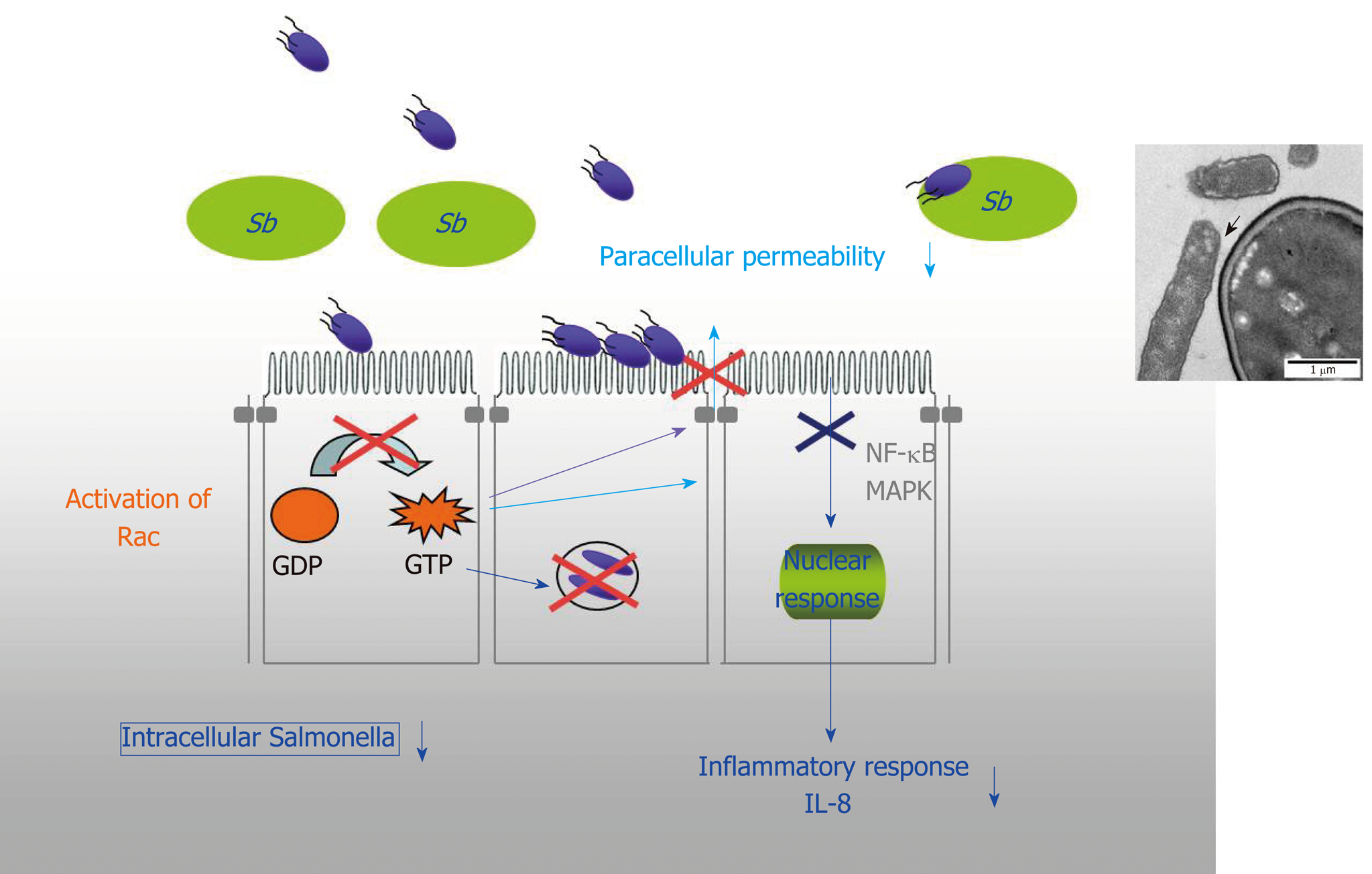
Figure 5 The mechanisms of protection against enteroinvasive bacteria (Salmonella and Shigella).
In the case of Salmonella, there is a decrease in the activation of the small GTPase pathway and consequently a decrease in the number of intracellular bacteria and the maintence of tight junctions. Saccharomyces boulardii CNCM I-745 also induces inhibition of mitogen-activated protein kinases and NF-κB activation pathways that are involved in interleukin 8 synthesis. And finally, a direct effect of S. boulardii CNCM I-745 on bacteria with the modification of their motility and the adhesion of Salmonella to S. boulardii CNCM I-745. IL-8: Interleukin 8; MAPK: Mitogen-activated protein kinase; Sb: Saccharomyces boulardii CNCM I-745.
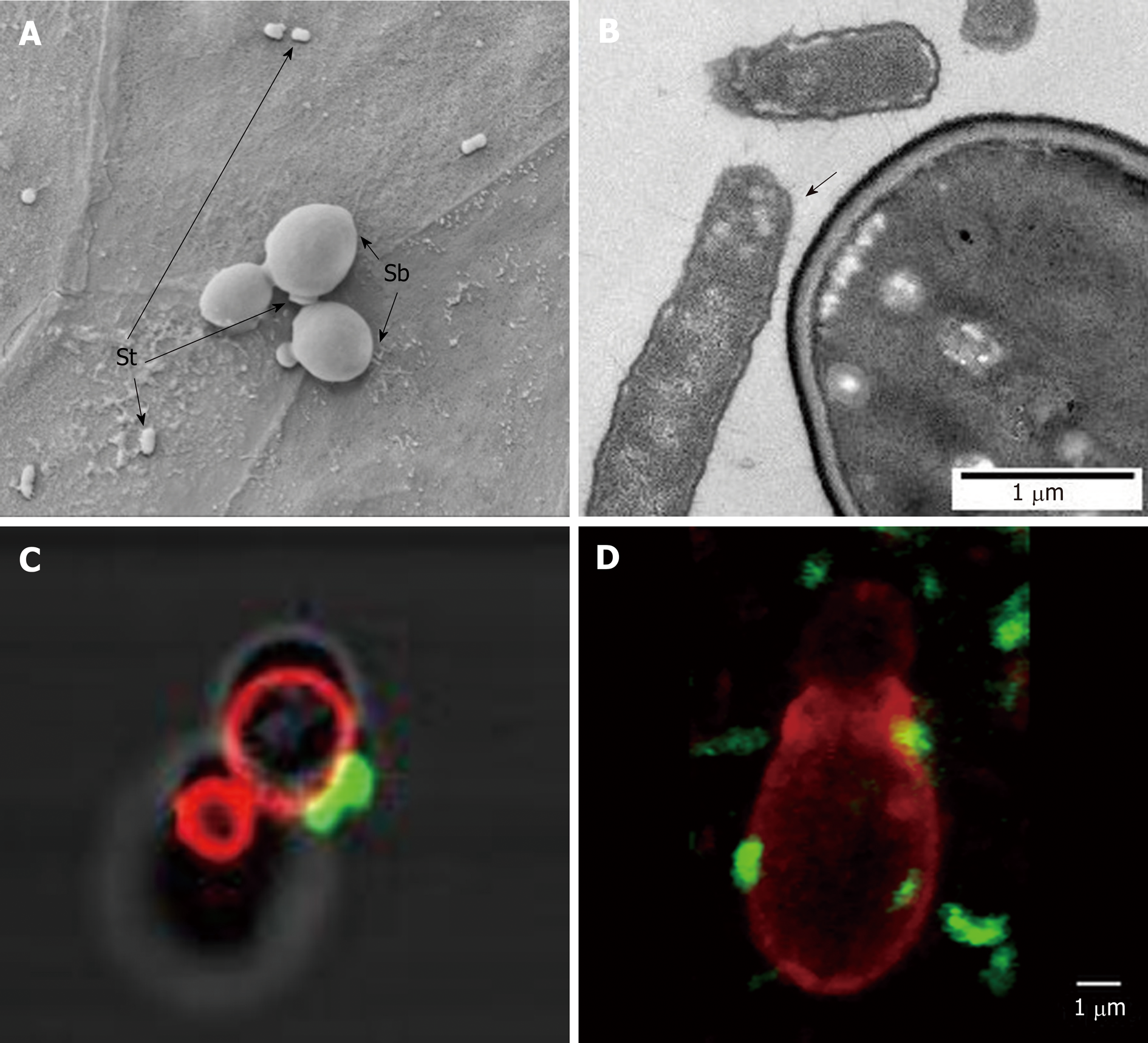
Figure 6 Scanning electron microscopy image showing Saccharomyces boulardii CNCM I-745 and Saccharomyces typhimurium on a monolayer of T84 polarized cells.
A and B: Electron microscopy image showing Saccharomyces typhimurium adhesion to Saccharomyces boulardii CNCM I-745; C and D: Confocal microscopy images showing S. typhimurium (Fluorescein IsoThioCyanate labelling), which adheres to S. boulardii CNCM I-745 (rhodamine labeling) in vitro (C) and in vivo on mouse cecum sections (D). Photos A and B: D. Czerucka1, P. Gounon2, P. Rampal1; C and D: D. Czerucka1, R. Pontier-Bres1, P. Rampal1 (1CSM, Monaco, microscopy Platform Cote d’Azur, MICA; 2University of Nice-Sophia-Antipolis). Sb: Saccharomyces boulardii CNCM I-745; St: Salmonella typhimurium.
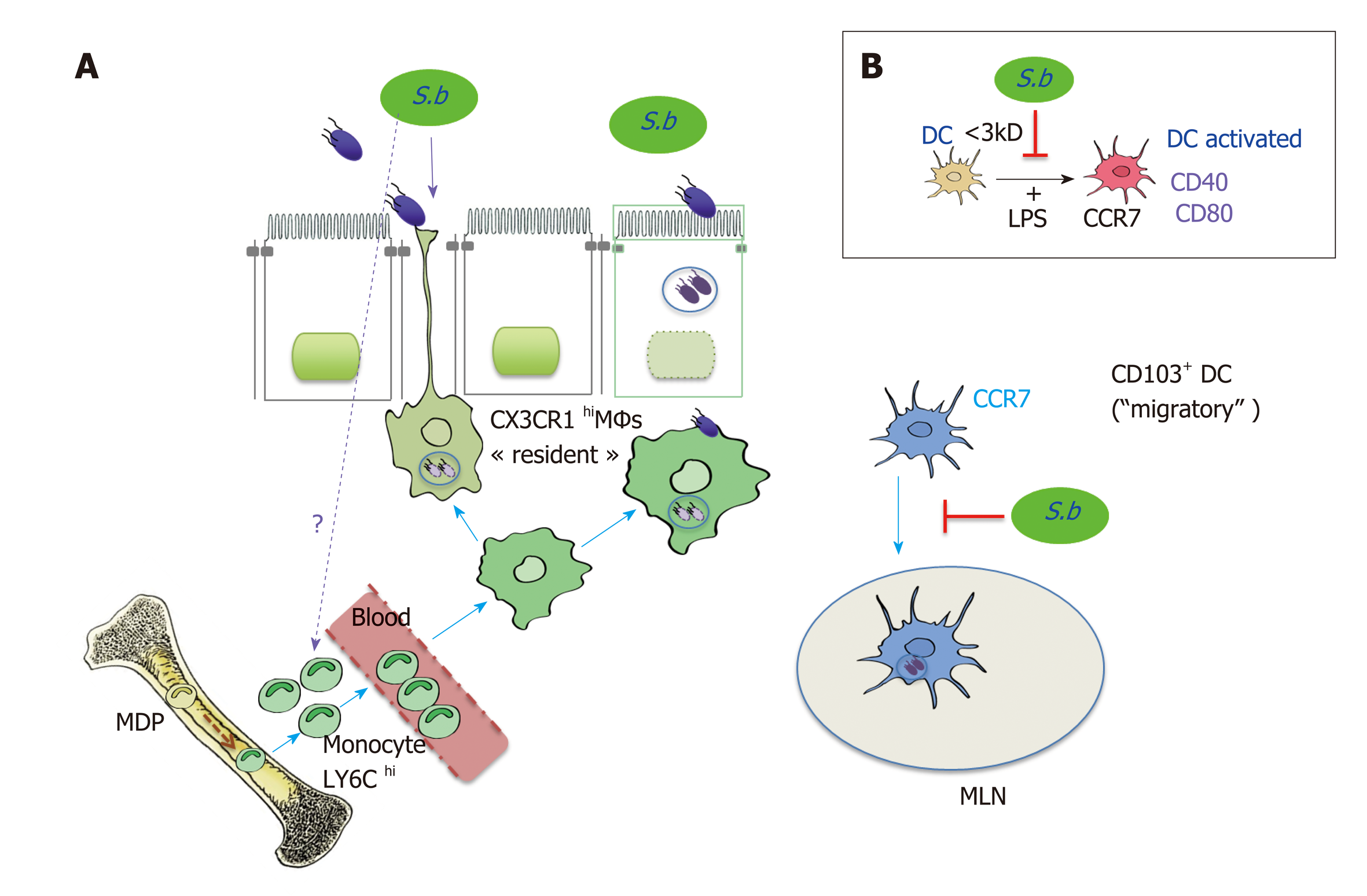
Figure 7 Effect of Saccharomyces boulardii CNCM I-745 on intestinal mononuclear phagocytes: Dendritic cells expressing CD103 (CD103+DC) and macropahge expressing the fractalkine receptor (CX3CR1MΦs).
A.CD103+DC which expresses the CCR7 on their surface phagocytes the Salmonella (ST) and migrate to the mesenteric lymph nodes (MLN). CX3CR1MΦs which have a high phagocytosis capacity, include bacteria, do not migrate, but remain in the LP where they stimulate T lymphocytes. These MΦs are able to form extensions that pass between the epithelial cells and capture the antigens in the intestinal lumen, among other pathogenic bacteria such as ST. Saccharomyces boulardii CNCM I-745 induces the recruitment of CX3CR1MΦs and promotes phagocytosis of ST by these cells. S. boulardii CNCM I-745 effects the expansion of Ly6C inflammatory monocytes, which are the precursors of CX3CR1 DCs in the bone marrow. In addition, S. boulardii CNCM I-745 reduces the number of ST that migrate to MLN by decreasing the number of migratory DCs. B.In vitro studies have shown that S. boulardii CNCM I-745 can modify lipopolysaccharide activation of migratory DCs. This effect would be due to a molecule of low molecular weight (< 3 kDa) present in S. boulardii CNCM I-745 conditioned medium[64]. Sb: Saccharomyces boulardiiCNCM I-745; ST: Salmonella; MLN: Mesenteric lymph nodes; DCs: Dendritic cells; LPS: Lipopolysaccharide.
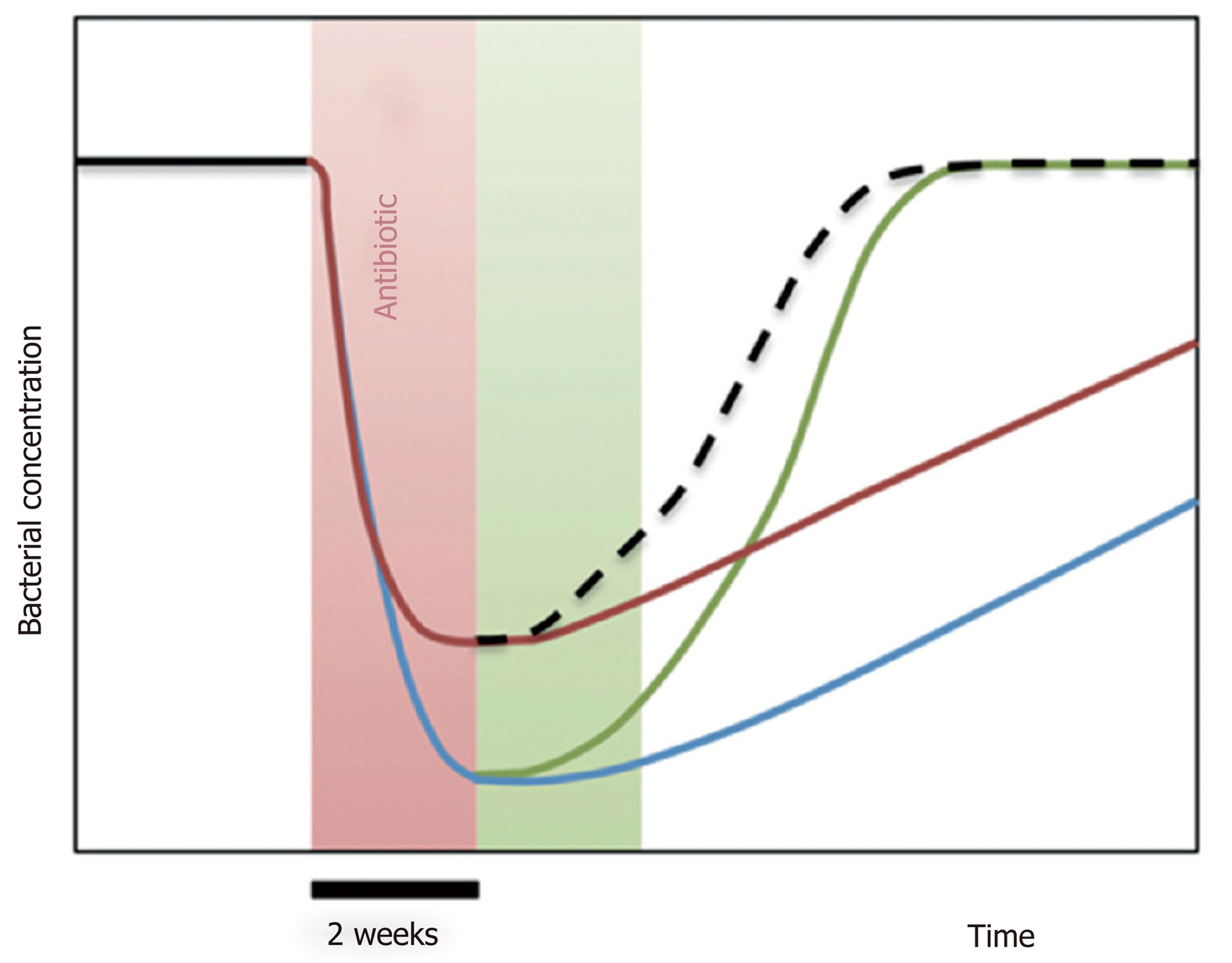
Figure 8 Diagram illustrating the impact of Saccharomyces boulardii CNCM I-745 on dysbiosis during antibiotic therapy.
A 2-wk treatment with antibiotics (red zone of the graph) induces a sudden decrease in the dominant bacterial populations of the microbiota (blue curve). Treatment with Saccharomyces boulardii CNCM I-745 during antibiotic therapy (red curve) reduces the sudden decrease in bacterial populations. When S. boulardii CNCM I-745 is administered after antibiotic therapy (green zone of the graph, green curve) the yeast accelerates the restoration of the intestinal flora to its initial level. An optimal use of yeast would be administration during and after antibiotic therapy, which is presented by the hatched curve resulting from the red and green curve (from reference[75]).
- Citation: Czerucka D, Rampal P. Diversity of Saccharomyces boulardii CNCM I-745 mechanisms of action against intestinal infections. World J Gastroenterol 2019; 25(18): 2188-2203
- URL: https://www.wjgnet.com/1007-9327/full/v25/i18/2188.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i18.2188