Copyright
©The Author(s) 2019.
World J Gastroenterol. Apr 28, 2019; 25(16): 1950-1963
Published online Apr 28, 2019. doi: 10.3748/wjg.v25.i16.1950
Published online Apr 28, 2019. doi: 10.3748/wjg.v25.i16.1950
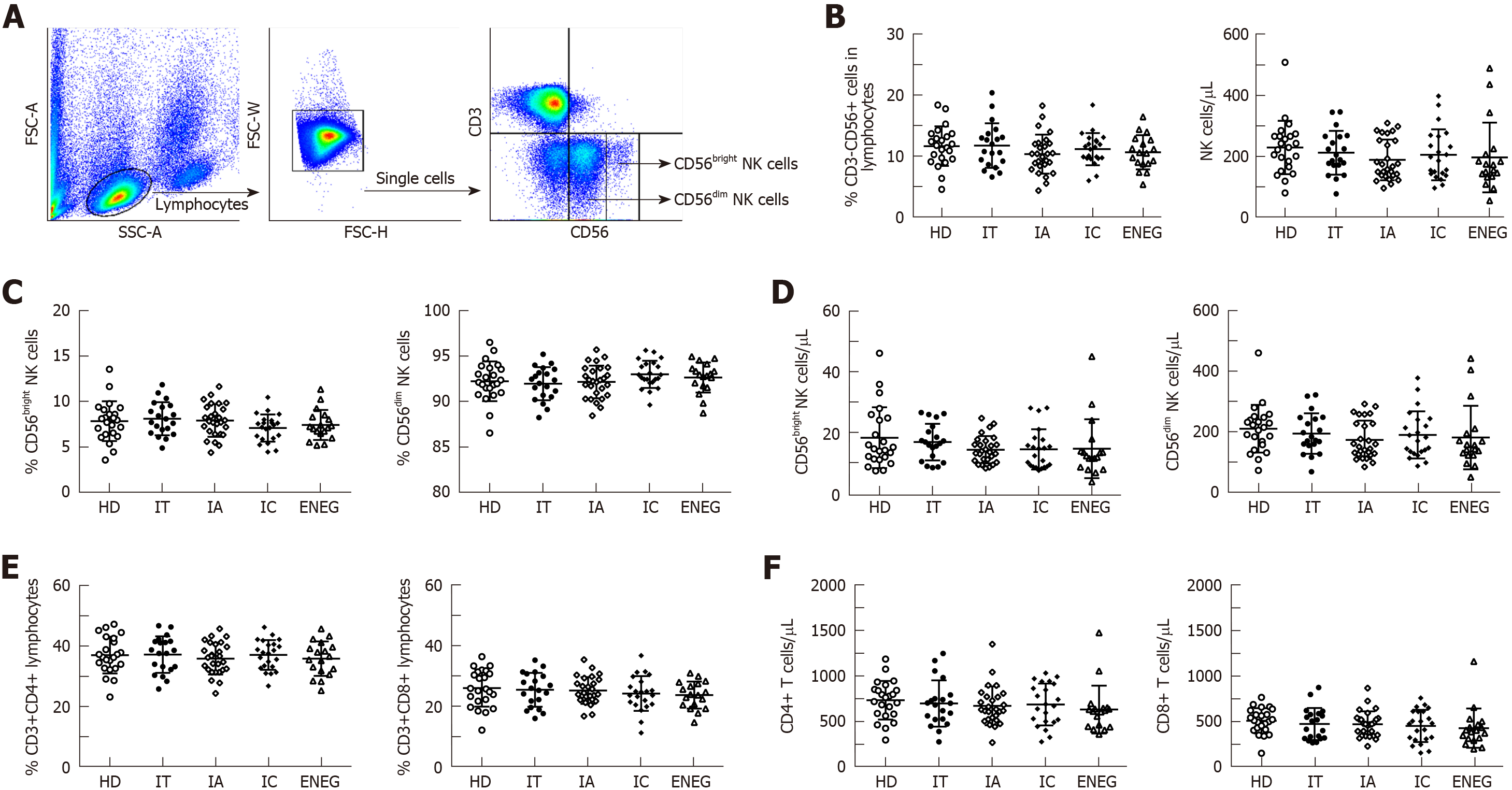
Figure 1 Frequencies and absolute number of NK cells, CD4+ and CD8+ T cells in different clinical phases.
A: Representative dot plots depicting the gating strategy for CD3-CD56+ NK cells, CD56bright and CD56dim subsets; B: Frequencies and absolute number of circulating NK cells in different phases; C: Frequencies of CD56bright and CD56dim NK cell subsets in different phases; D: Absolute number of CD56bright and CD56dim NK cell subsets in different phases; E: Frequencies of CD4+ and CD8+ T cells in lymphocytes in different phases; F: Absolute number of CD4+ and CD8+ T cells in different phases. All data are presented as mean ± SD. NK: Natural killer; HD: Healthy honors; IT: Immune tolerant; IA: Immune active; IC: Inactive carrier; ENEG: Hepatitis B envelope antigen-negative hepatitis.
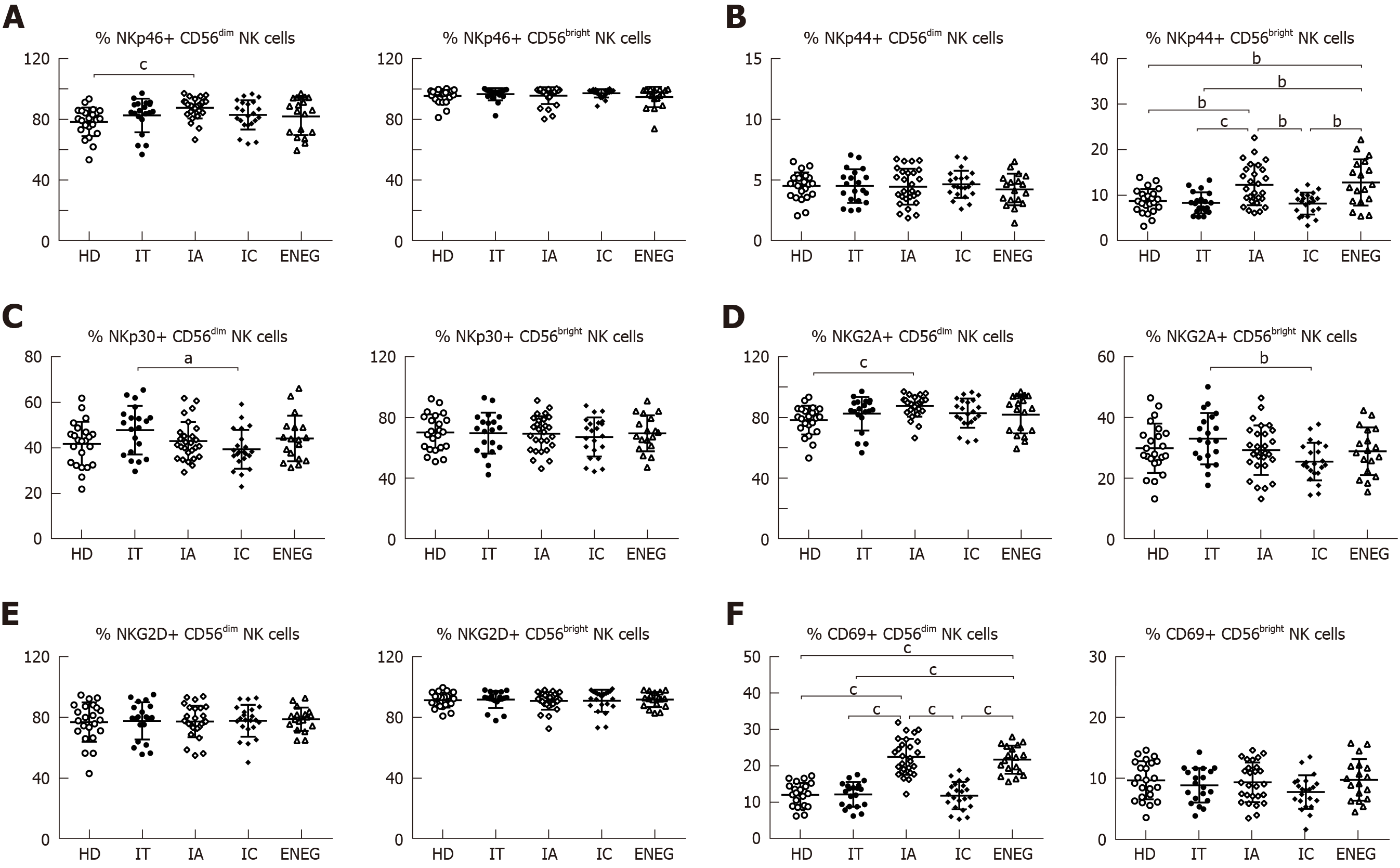
Figure 2 Phenotypes of NK cells in different clinical phases.
A: Frequency of CD56bright and CD56dim NK cells expressing the activating receptor NKp46; B: Frequency of CD56bright and CD56dim NK cells expressing the activating receptor NKp44; C: Frequency of CD56bright and CD56dim NK cells expressing the activating receptor NKp30; D: Frequency of CD56bright and CD56dim NK cells expressing the inhibitory receptor NKG2A; E: Frequency of CD56bright and CD56dim NK cells expressing the activating receptor NKG2D; F: Frequency of CD56bright and CD56dim NK cells expressing the activating marker CD69. NK: Natural killer; HD: Healthy honors; IT: Immune tolerant; IA: Immune active; IC: Inactive carrier; ENEG: Hepatitis B envelope antigen-negative hepatitis. All data are presented as mean ± SD, aP < 0.05, bP < 0.01, cP < 0.001.
Figure 3 NK cell functions in different clinical phases.
For detecting NK cell functions, peripheral blood mononuclear cells were stimulated by IL-12 and IL-18, P/I, K562 target cells or IL-12/18 and K562 target cells. A: Gated on CD3-CD56+ NK cells, representative dot plots depicting the expression of IFN-γ, TNF-α, CD107a, perforin and granzyme B in NK cells; B: Pooled data showing the frequency of NK cells expressing IFN-γ and TNF-α at different phases; C: Pooled data showing the differences in NK cell CD107a degranulation in different phases; D: Summary data showing the frequency of NK cells producing perforin and granzyme B in different phases. IFN-γ: Interferon-gamma; TNF-α: Tumor necrosis factor-alpha; NK: Natural killer; HD: Healthy honors; IT: Immune tolerant; IA: Immune active; IC: Inactive carrier; ENEG: Hepatitis B envelope antigen-negative hepatitis; P/I: Phorbol myristate acetate and ionomycin. All data are presented as mean ± SD, aP < 0.05, bP < 0.01, cP < 0.001.
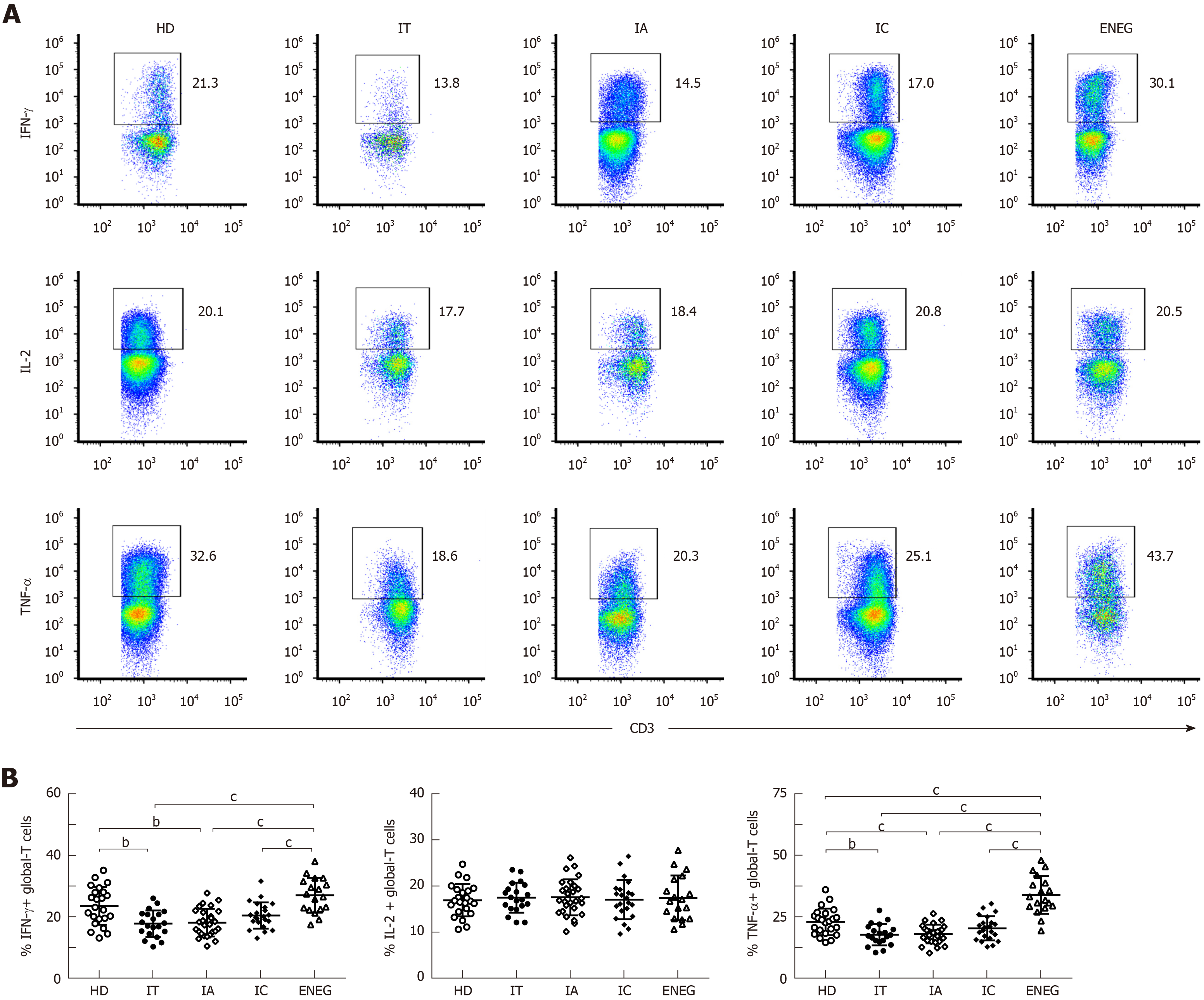
Figure 4 Global-T cell cytokine production in different clinical phases.
Peripheral blood mononuclear cells were stimulated by phorbol myristate acetate and ionomycin. A: Representative dot plots depicting the expression of IFN-γ, IL-2 and TNF-α in global CD3+ T cells; B: Cumulative data showing the frequency of global CD3+ T cells expressing IFN-γ, IL-2 and TNF-α in healthy donors and patients in different clinical phases. IFN-γ: Interferon-gamma; TNF-α: Tumor necrosis factor-alpha; HD: Healthy donors; IT: Immune tolerant; IA: Immune active; IC: Inactive carrier; ENEG: Hepatitis B envelope antigen-negative hepatitis. All data are presented as mean ± SD, aP < 0.05, bP < 0.01, cP < 0.001.
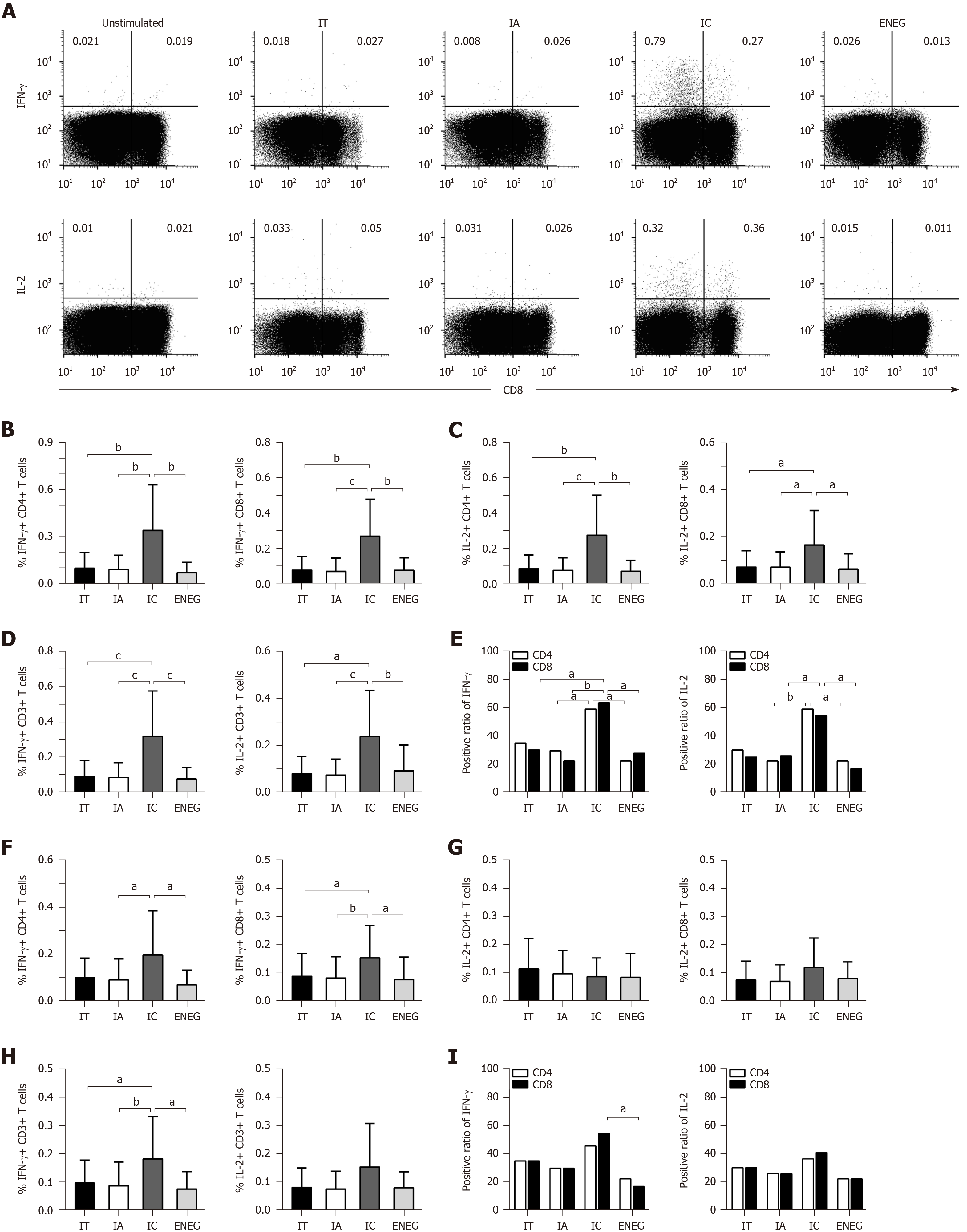
Figure 5 Virus-specific T cell responses after in vitro expansion in different clinical phases.
Peripheral blood mononuclear cells were incubated with core or S peptide pools. After 10 d in vitro culture, virus-specific T cell responses were determined by detecting the frequency of T cells producing interferon-gamma (IFN-γ) and IL-2. A: Gated on CD3+ lymphocytes, representative dot plots depict the frequency of CD4+ (upper-left quadrant) and CD8+ (upper-right quadrant) T cells-producing IFN-γ or IL-2; B: IFN-γ production by CD4+ and CD8+ T cells in response to the core peptide pool; C: IL-2 production by CD4+ and CD8+ T cells in response to the core peptide pool; D: IFN-γ and IL-2 production by CD3+ T cells in response to the core peptide pool; E: Positive responses of CD4+ or CD8+ T cells producing IFN-γ or IL-2 in response to the core peptide pool; F: IFN-γ production by CD4+ and CD8+ T cells in response to the S peptide pool; G: IL-2 production by CD4+ and CD8+ T cells in response to the S peptide pool; H: IFN-γ and IL-2 production by CD3+ T cells in response to the S peptide pool; I: Positive responses of CD4+ or CD8+ T cells producing IFN-γ or IL-2 in response to the S peptide pool. IFN-γ: Interferon-gamma; TNF-α: Tumor necrosis factor-alpha; HD: Healthy donors; IT: Immune tolerant; IA: Immune active; IC: Inactive carrier; ENEG: Hepatitis B envelope antigen-negative hepatitis. All data are presented as mean ± SD, aP < 0.05, bP < 0.01, cP < 0.001.
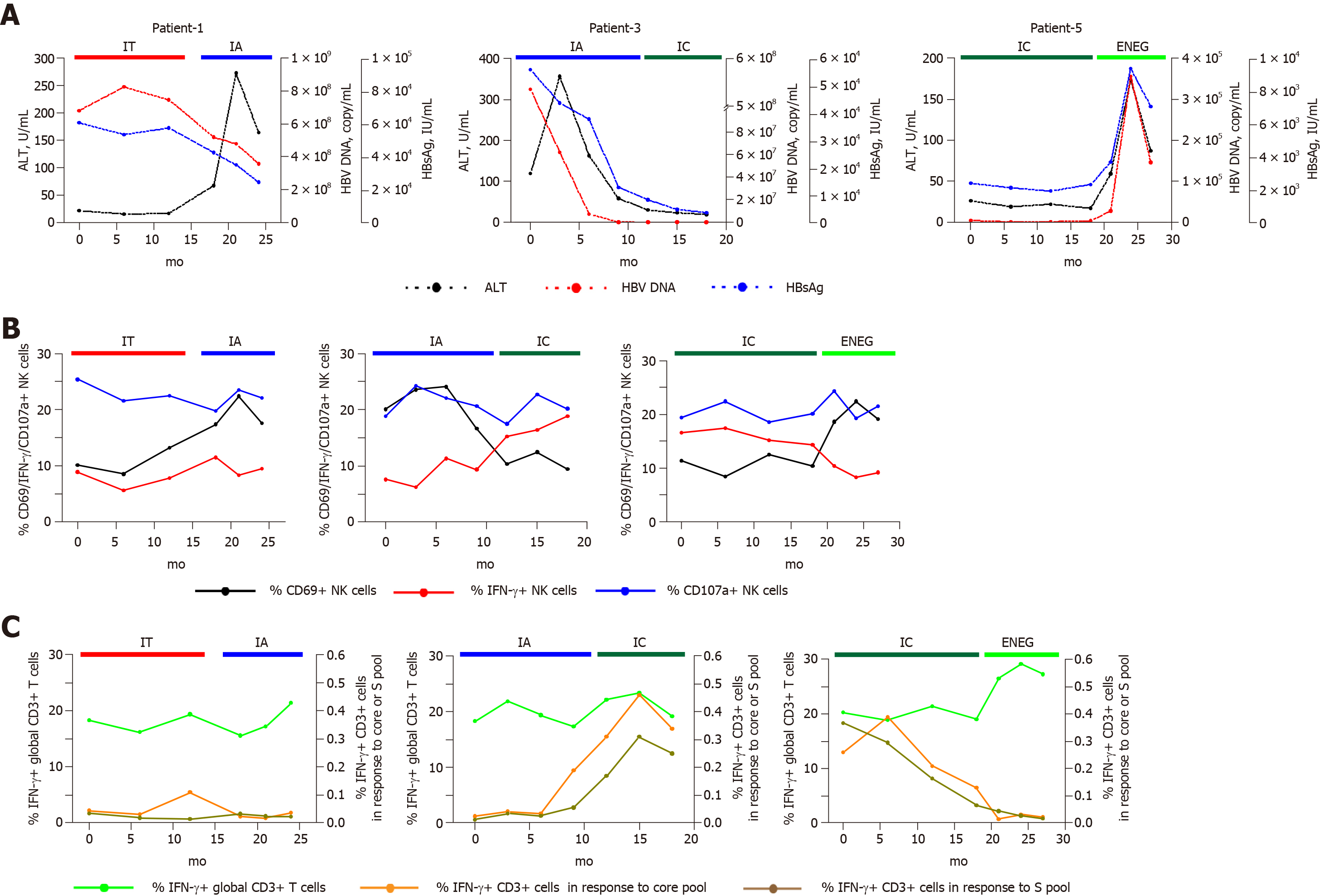
Figure 6 Longitudinal analysis of NK and T cell responses in representative individuals who transitioned from one phase to another.
Patient-1, a patient who transitioned from the immune tolerant to immune active phase. Patient-3, a patient who experienced spontaneous HBeAg clearance. Patient-5, a patient in the clinical phase changed from inactive carrier to HBeAg-negative hepatitis phase. A: Dynamic fluctuations of clinical parameters from one phase to another, including alanine aminotransferase, hepatitis B virus DNA and hepatitis B surface antigen; B: Phenotypic and functional changes of natural killer cells in representative patients who transitioned from one phase to another; C: Changes of interferon-gamma production in global T cells, and core or S peptide pool-stimulated T cells. NK: Natural killer; HBV: Hepatitis B virus; HBsAg: Hepatitis B surface antigen; HBeAg: Hepatitis B envelope antigen; ALT: Alanine aminotransferase; IFN-γ: Interferon-gamma; TNF-α: Tumor necrosis factor-alpha; HD: Healthy honors; IT: Immune tolerant; IA: Immune active; IC: Inactive carrier; ENEG: Hepatitis B envelope antigen-negative hepatitis.
- Citation: Wang WT, Zhao XQ, Li GP, Chen YZ, Wang L, Han MF, Li WN, Chen T, Chen G, Xu D, Ning Q, Zhao XP. Immune response pattern varies with the natural history of chronic hepatitis B. World J Gastroenterol 2019; 25(16): 1950-1963
- URL: https://www.wjgnet.com/1007-9327/full/v25/i16/1950.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i16.1950