Copyright
©The Author(s) 2018.
World J Gastroenterol. Sep 28, 2018; 24(36): 4132-4151
Published online Sep 28, 2018. doi: 10.3748/wjg.v24.i36.4132
Published online Sep 28, 2018. doi: 10.3748/wjg.v24.i36.4132
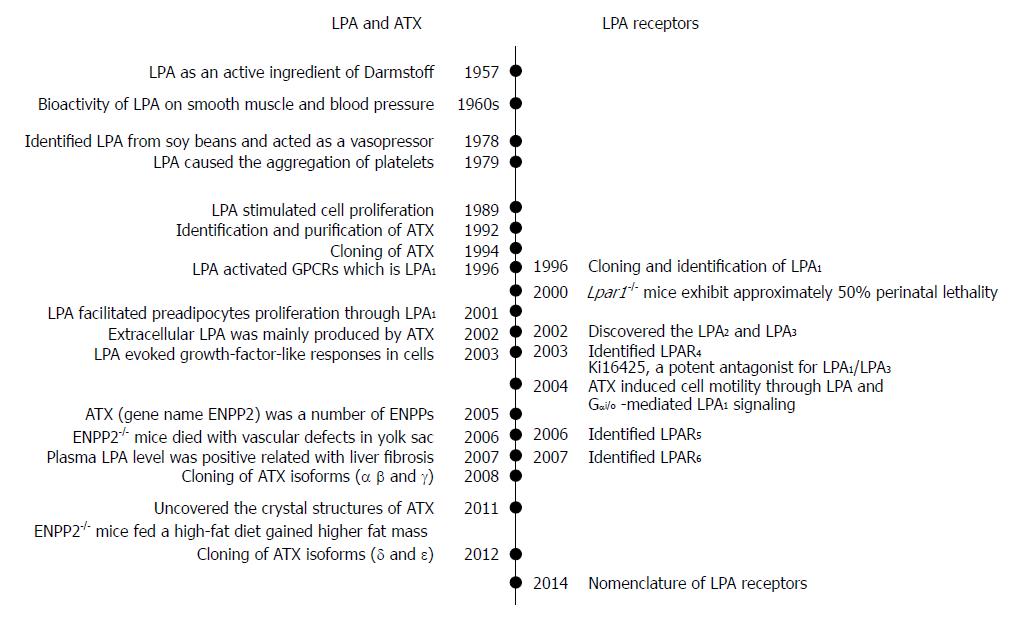
Figure 1 Chronological events related the identifications of lysophosphatidic acid, ecto-nucleotide pyrophosphatase/phosphodiesterase/autotaxin and lysophosphatidic acid receptors.
On the left side, it shows the events associated with the identifications of LPA molecules and ATX for its production. On the right side, it shows the cloning events of LPAR1-6. LPA: Lysophosphatidic acid; ATX: Autotaxin; LPA1-6: Lysophosphatidic acid receptor 1-6; ENPP2: Ectonucleotide pyrophosphatase/phosphodiesterase family member 2.
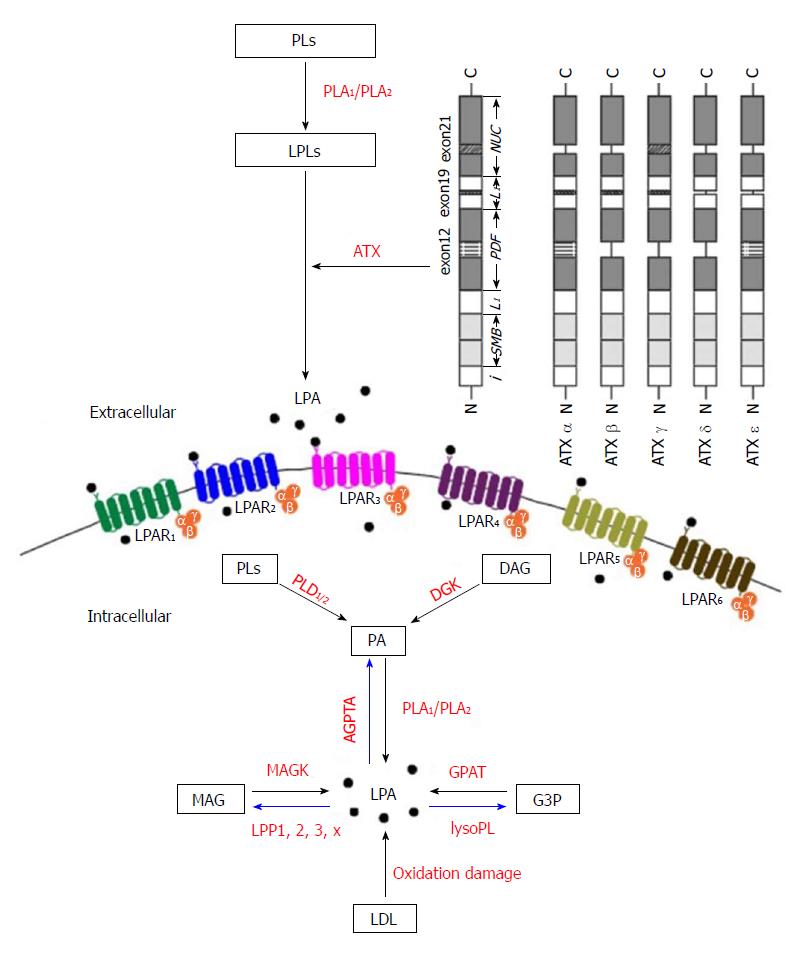
Figure 2 Biochemical pathways of lysophosphatidic acid synthesis and degradation.
LPA can be produced extracellularly and intracellularly as signaling mediators and membrane components, respectively. There are five major pathways for LPA production, (1) the lysophospholipids-ATX (LPLs-ATX) pathway, (2) the phosphatidic acid - phospholipase A1 or A2 (PA-PLA1/PLA2) pathway, (3) the de novo glycerophosphate acyltransferase (GPAT) synthesis pathway, (4) the monoacylglycerol kinase (MAGK) pathway, and (5) the oxidative modification of low-density lipoprotein (LDL) pathway. In the upper right corner of the figure, there are catalytically active isoforms (ATXα, ATXβ, ATXγ, ATXδ and ATXε), which are expressed in different tissues. PLs: Phospholipids; PLA1/PLA2: Phospholipase A1/2; LPLs: Lysophospholipids; ATX: Autotaxin; ATXα-δ: Protein structure scheme of the domains of ATX; LPA: Lysophosphatidic acid; DAG: Diacylglycerol; DGK: Diacylglycerol kinase; PLD1/2: Phospholipase D1/2; PA: Phosphatidic acid; AGPAT: Acylglycerophosphate acyltransferase; MAG: Monoacylglycerol; MAGK: Monoacylglycerol kinase; LPP: Lipid phosphate phosphatase; G3P: Glycerol-3-phosphate; lyso PL: Lysophospholipase; GPAT: Glycerophosphate acyltransferase; LDL: Low-density lipoprotein; i: Intramembrane domain; SMB: N-terminal somatomedin B-like domains; L1: L1 linker region; PDF: Phosphodiesterase domain; L2: L2 linker region; NUC: C-terminal nuclease-like domain; LPA1-6: Lysophosphatidic acid receptor 1-6.
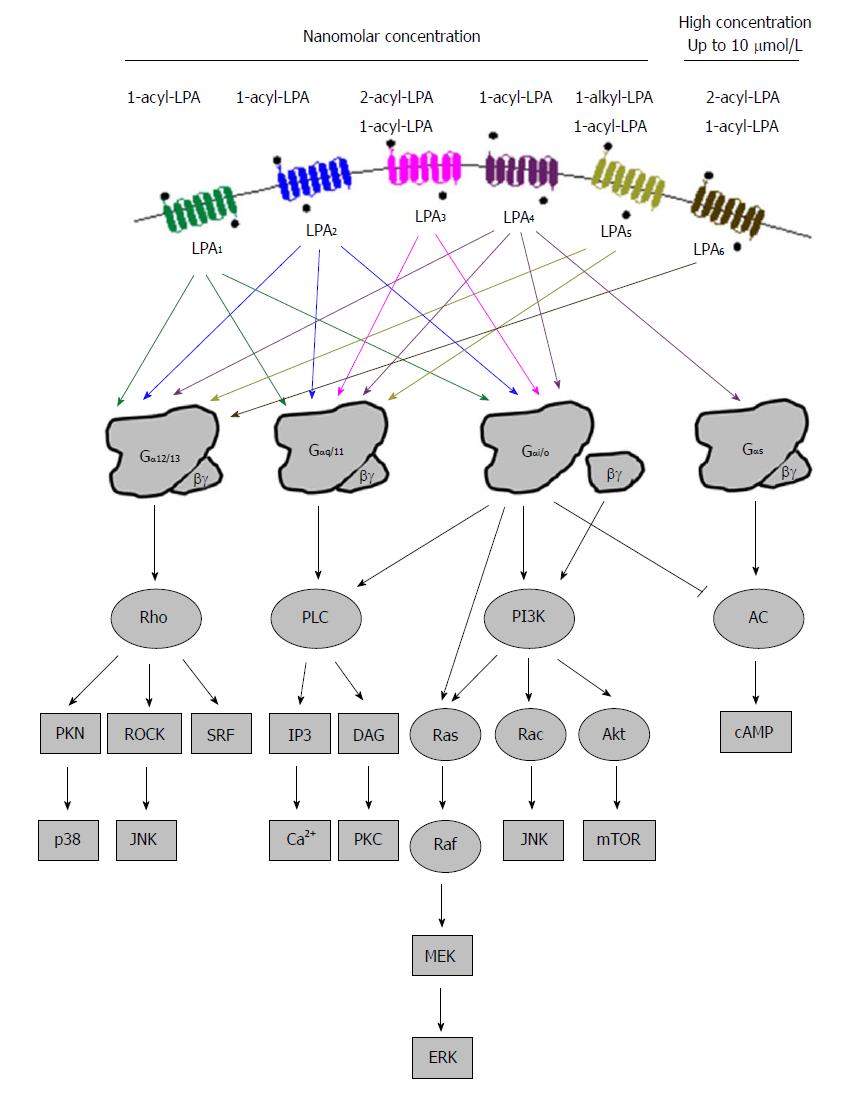
Figure 3 Summary of lysophosphatidic acid activated intracellular signaling pathways via the six cognate lysophosphatidic acid receptors.
PLC: Phospholipase C; PI3K: Phosphatidylinositol 3-kinase; AC: Denylyl cyclase; PNK: Polynucleotide 5'-hydroxyl-kinase; ROCK: Rho-associated kinase; JNK: c-jun N-terminal kinase; SRF: Serum response factor; IP3: Inositol 1,4,5-triphosphate; DAG: Diacylglyerol; PKC: Protein kinase C; MEK: Mitogen-activated protein kinase; ERK: Extracellular signal-regulated kinase; Akt: Protein kinase B; Mtor: Mammalian target of rapamycin.
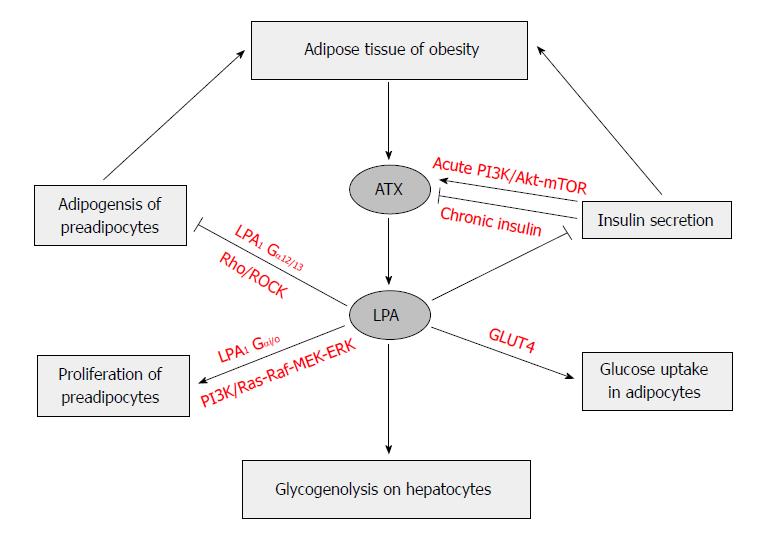
Figure 4 Autotaxin-lysophosphatidic acid signaling axis regulates adipose tissue development and glucose homeostasis in obesity.
In adipose tissue, especially in mature adipocytes, the elevated expression of AXT leads to production of LPA and then induced proliferation of preadipocytes via LPA1 through Ras-Raf-MEK-ERK pathway. On the other hand, LPA inhibits differentiation of white and brown preadipocytes, which is mediated by LPA1via the Rho-ROCK pathway. Short-term insulin treatment increases ATX secretion in adipocytes via PI3K/Akt-mTOR pathway, whereas long-term insulin treatment reduces ATX activity. LPA produced by ATX in obesity has a tonic inhibitory effect on glucose homeostasis through inhibition of insulin secretion in isolated pancreas islets, increase of glucose transport in myocyte and adipocytes via GLUT4 translocation in a PI3K dependent manner, and elevation of glycogenolysis in hepatocytes. ROCK: Rho-associated kinase; PI3K: Phosphatidylinositol 3-kinase; MEK: Mitogen-activated protein kinase; ERK: Extracellular signal-regulated kinase; Akt: Protein kinase B; mTOR: Mammalian target of rapamycin; GLUT4: Glucose transporter type 4.
- Citation: Yang F, Chen GX. Production of extracellular lysophosphatidic acid in the regulation of adipocyte functions and liver fibrosis. World J Gastroenterol 2018; 24(36): 4132-4151
- URL: https://www.wjgnet.com/1007-9327/full/v24/i36/4132.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i36.4132