Copyright
©The Author(s) 2017.
World J Gastroenterol. Dec 21, 2017; 23(47): 8415-8425
Published online Dec 21, 2017. doi: 10.3748/wjg.v23.i47.8415
Published online Dec 21, 2017. doi: 10.3748/wjg.v23.i47.8415
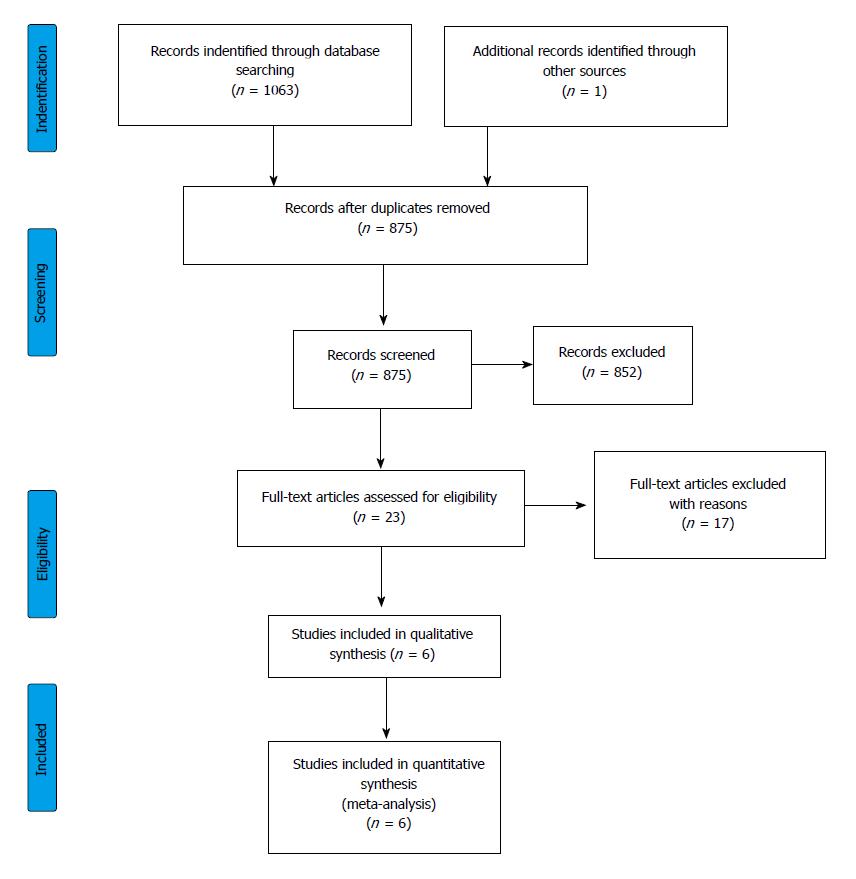
Figure 1 Flowchart of the study selection procedure.
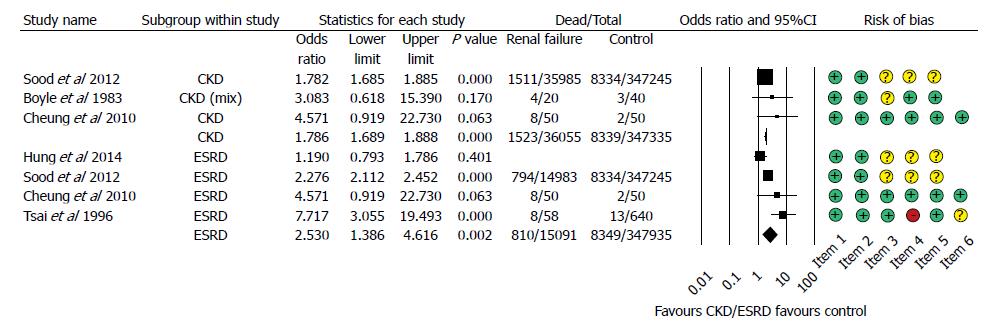
Figure 2 Forest plot representing the differences in mortality in gastrointestinal bleeding patients with normal and impaired renal function.
Size of squares for risk ratio reflects weight of trial in pooled analysis. Horizontal bars represent 95%CI. CKD: Chronic kidney disease; ESRD: End-stage renal disease.
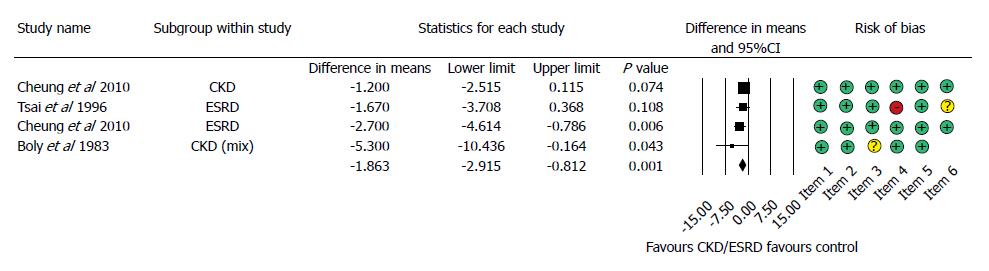
Figure 3 Forest plot representing the required units of transfusion in gastrointestinal bleeding patients with normal and impaired renal function.
Size of squares for the difference in standardized mean values reflects weight of trial in pooled analysis. Horizontal bars represent 95%CI. CKD: Chronic kidney disease; ESRD: End-stage renal disease.
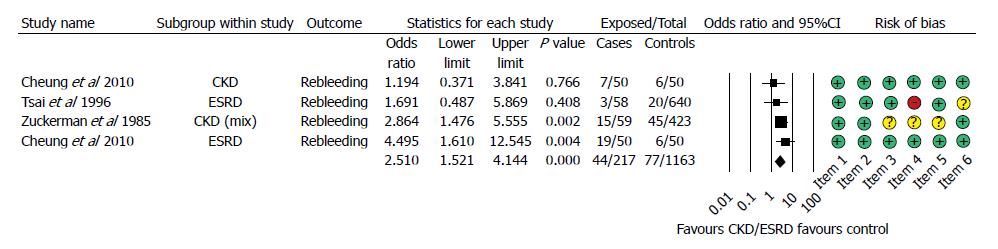
Figure 4 Forest plot representing the rebleeding rate in gastrointestinal bleeding patients with normal and impaired renal function.
Size of squares for risk ratio reflects weight of trial in pooled analysis. Horizontal bars represent 95%CI. CKD: Chronic kidney disease; ESRD: End-stage renal disease.
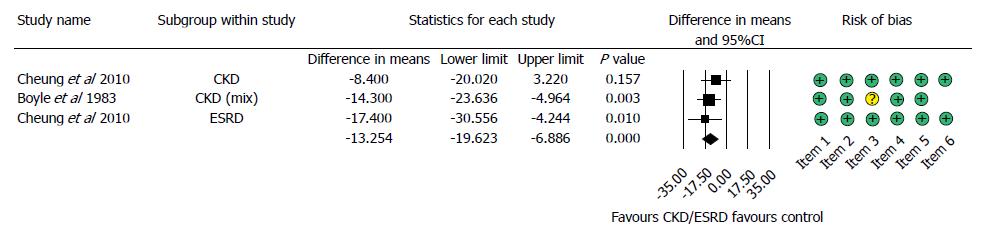
Figure 5 Forest plot representing the differences in length of hospitalization in gastrointestinal bleeding patients with normal and impaired renal function.
Size of squares for the difference in standardized mean values reflects weight of trial in pooled analysis. Horizontal bars represent 95%CI. CKD: Chronic kidney disease; ESRD: End-stage renal disease.
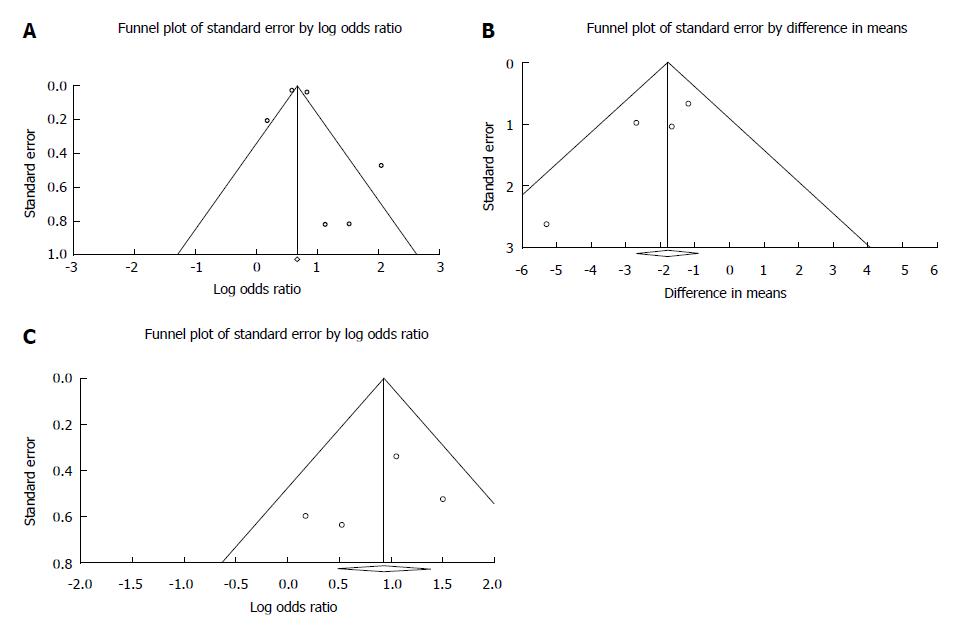
Figure 6 Funnel plot.
A: Funnel plot of mortality; B: Funnel plot of required transfusion; and C: Funnel plot of rebleeding.
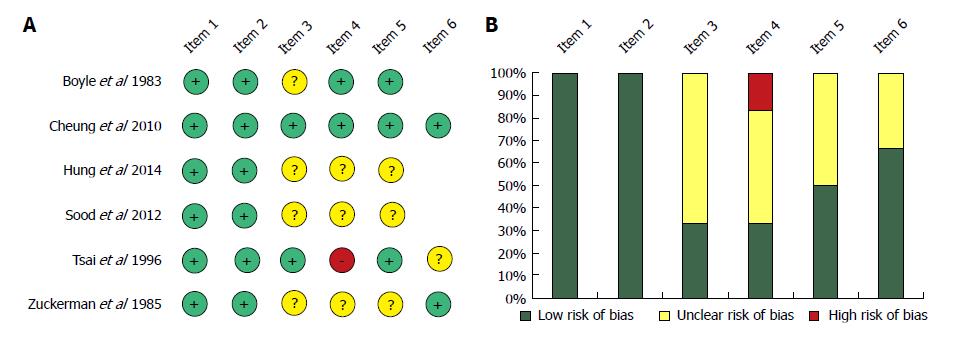
Figure 7 Risk assessment of articles included in the meta-analysis based on the modified Newcastle-Ottawa Scale (A); Risk of bias assessment graph (B).
- Citation: Hágendorn R, Farkas N, Vincze Á, Gyöngyi Z, Csupor D, Bajor J, Erőss B, Csécsei P, Vasas A, Szakács Z, Szapáry L, Hegyi P, Mikó A. Chronic kidney disease severely deteriorates the outcome of gastrointestinal bleeding: A meta-analysis. World J Gastroenterol 2017; 23(47): 8415-8425
- URL: https://www.wjgnet.com/1007-9327/full/v23/i47/8415.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i47.8415