Copyright
©The Author(s) 2017.
World J Gastroenterol. Dec 21, 2017; 23(47): 8308-8320
Published online Dec 21, 2017. doi: 10.3748/wjg.v23.i47.8308
Published online Dec 21, 2017. doi: 10.3748/wjg.v23.i47.8308
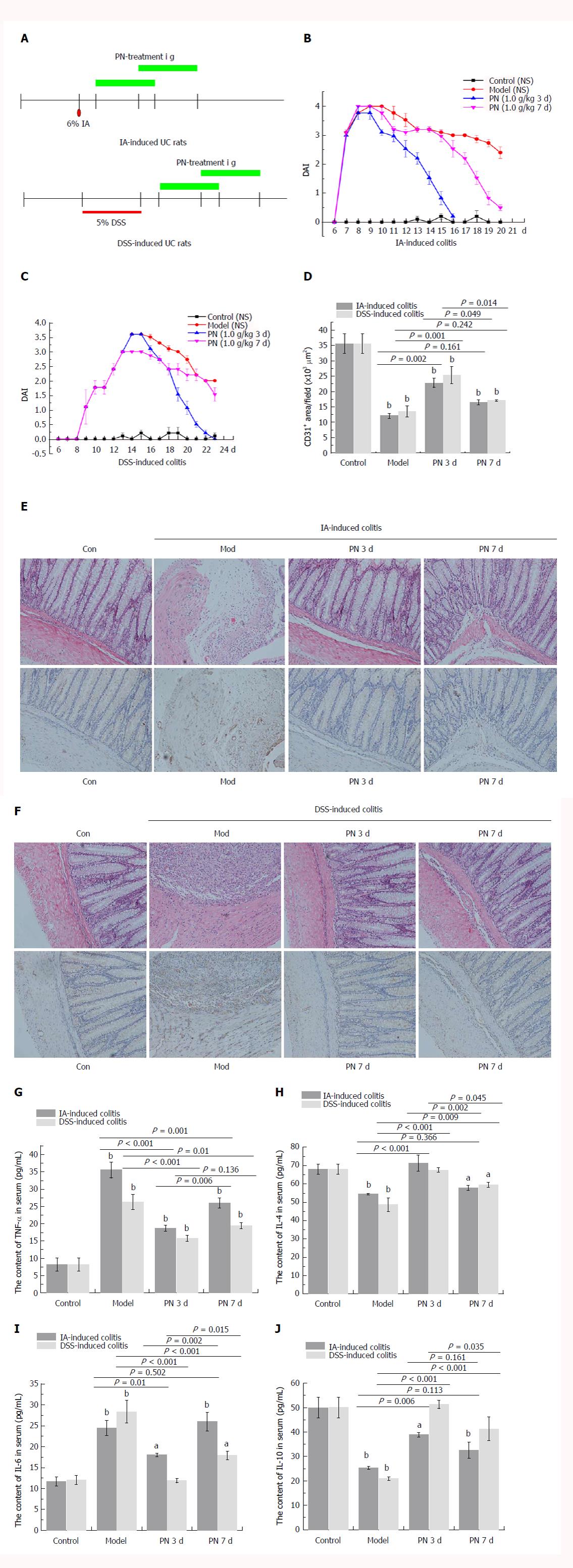
Figure 1 Efficacy of Panax notoginseng in experimental colitis was dependent on initial time of administration.
A: PN administration (1.0 g/kg) was initiated at day 3 and 7 for seven consecutive days; B, C, G, J: DAI scores and serum concentrations of TNF-α and IL-6 in the day 3 group were significantly lower than those in the day 7 group; D, E: The pathological lesions of colonic mucosa and microvessels in the day 3 group were less than those in the day 7 group; F: MVD in the day 3 group was significantly higher than that in the day 7 group. Results are expressed as mean ± SD of three independent experiments performed in triplicate. aP < 0.05, bP < 0.01 vs normal control. DAI: Disease activity index; IL: Interleukin; MVD: Microvessel density; PN: Panax notoginseng; TNF: Tumor necrosis factor.
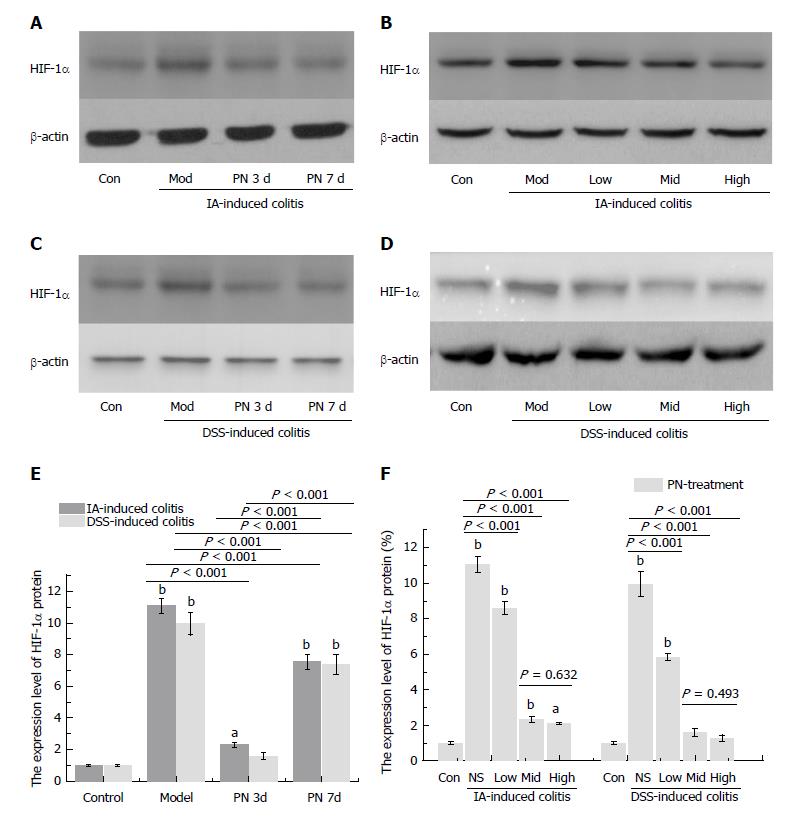
Figure 2 Panax notoginseng improved hypoxia in colonic mucosa.
A-F: Increased expression of hypoxia-inducible factor-1α in colonic mucosa of the experimental colitis group was down-regulated by PN in a time- and dose-dependent manner. Results are expressed as mean ± SD of three independent experiments performed in triplicate. aP < 0.05, bP < 0.01 vs normal control. PN: Panax notoginseng.
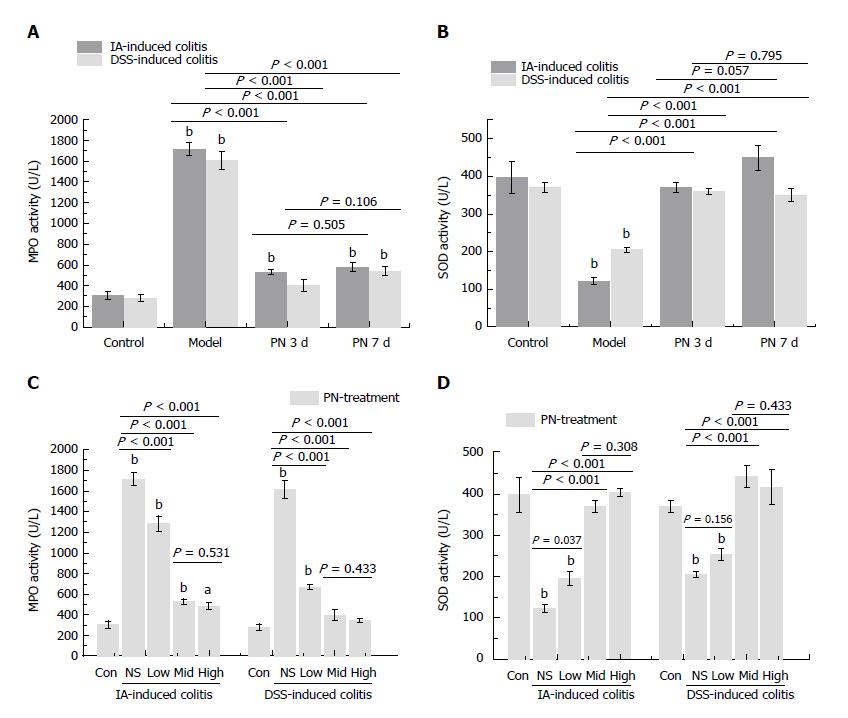
Figure 3 Panax notoginseng blocked oxidative stress in colonic mucosa.
Activities of MPO and SOD in colonic tissue were used to evaluate the anti-oxidative effect of PN. A-D: The increased activity of MPO and decreased activity of SOD in the experimental colitis groups were reversed by PN in a time- and dose-dependent manner. Results are expressed as mean ± SD of three independent experiments performed in triplicate. aP < 0.05, bP < 0.01 vs normal control. MPO: Myeloperoxidase; PN: Panax notoginseng; SOD: Superoxide dismutase.
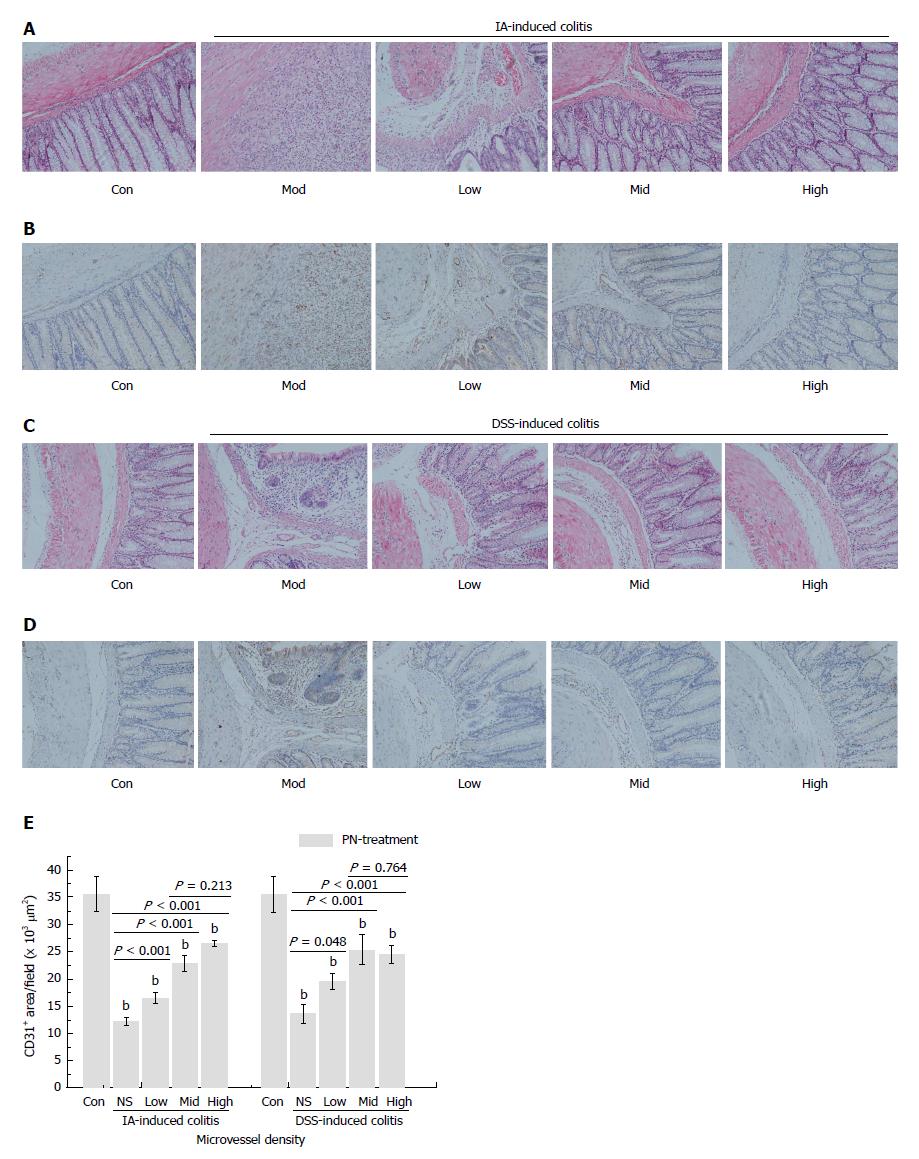
Figure 4 Panax notoginseng repaired colonic mucosal injuries and microvessels.
IA- and DSS-induced experimental colitis groups were treated with PN (0.5, 1.0 and 2.0 g/kg) for seven consecutive days. A, C: Colonic mucosal injuries and microvessels significantly improved; B, D, E: Microvessel density increased compared with the control groups in a dose-dependent manner. Results are expressed as mean ± SD of three independent experiments performed in triplicate. bP < 0.01 vs normal control. DSS: Dextran sodium sulfate; IA: Iodoacetamide; PN: Panax notoginseng.
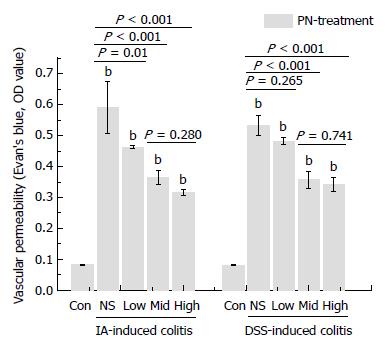
Figure 5 Panax notoginseng improved impaired vascular permeability.
A and B: Increased VP in the IA- and DSS-induced experimental colitis groups was decreased by medium and high doses of PN. Results are expressed as mean ± SD of three independent experiments performed in triplicate. bP < 0.01 vs normal control. DSS: Dextran sodium sulfate; IA: Iodoacetamide; PN: Panax notoginseng; VP: Vascular permeability.
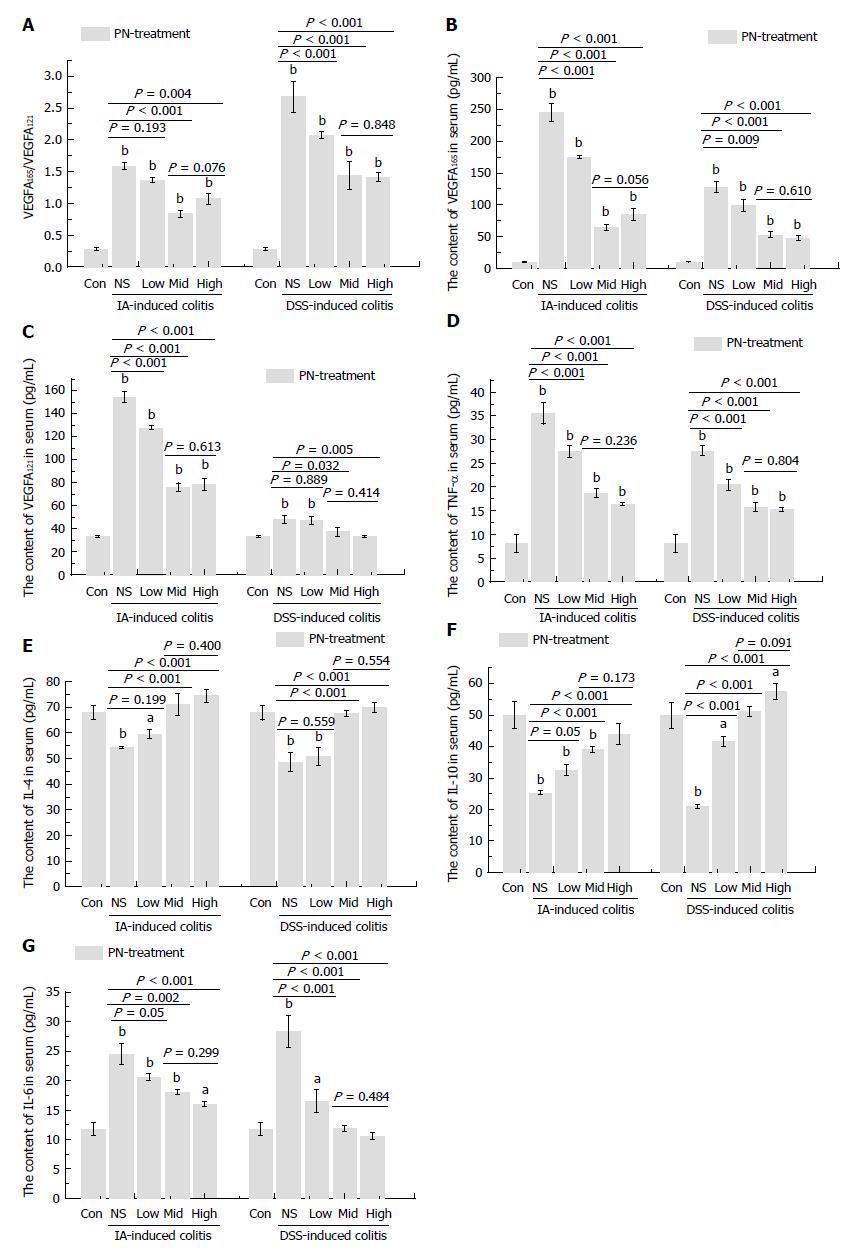
Figure 6 Panax notoginseng reversed the disordered ratio of VEGFA165/VEGFA121 and adjusted the imbalance of pro-inflammatory and anti-inflammatory cytokines.
A-C: Increased serum concentrations of VEGF165 and VEGF121, as well as the increased ratio of VEGF165/VEGF121 in the experimental colitis groups were down-regulated by medium and high doses of PN; D-F: The increased serum concentrations of IL-6 and TNF-α and decreased serum concentrations of IL-4 and IL-10 in the experimental colitis groups were reversed by PN in a dose-dependent manner. Results are expressed as mean ± SD of three independent experiments performed in triplicate. aP < 0.05, bP < 0.01 vs normal control. IL: Interleukin; PN: Panax notoginseng; TNF: Tumor necrosis factor; VEGF: Vascular endothelial growth factor.
- Citation: Wang SY, Tao P, Hu HY, Yuan JY, Zhao L, Sun BY, Zhang WJ, Lin J. Effects of initiating time and dosage of Panax notoginseng on mucosal microvascular injury in experimental colitis. World J Gastroenterol 2017; 23(47): 8308-8320
- URL: https://www.wjgnet.com/1007-9327/full/v23/i47/8308.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i47.8308