Copyright
©The Author(s) 2017.
World J Gastroenterol. Aug 7, 2017; 23(29): 5295-5303
Published online Aug 7, 2017. doi: 10.3748/wjg.v23.i29.5295
Published online Aug 7, 2017. doi: 10.3748/wjg.v23.i29.5295
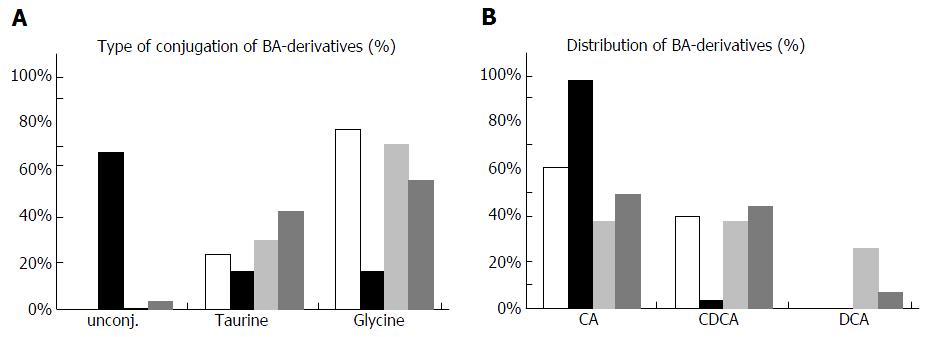
Figure 1 Bile acid derivatives in bile samples of the two patients.
Concentrations of different bile acid derivatives were measured in bile samples of two patients possessing the bile salt export pump (BSEP)-T919del and BSEP-G1032R mutation, respectively, as well as in six non-cholestatic and in six cholestatic control samples (Table 1). A: Relative amounts of unconjugated, taurine- or glycine-conjugated bile acids are shown. In the background of the BSEP-G1032R mutation a very high amount ( > 60%) of unconjugated bile salts is found; B: Relative amounts of cholic, chenodeoxycholic and deoxycholic acid (CA, CDCA, DCA) including their corresponding conjugates are shown. In the background of the BSEP-G1032R less than 5% of bile acids are chenodeoxycholic acid and its conjugates (UDCA and conjugates were omitted from these plots). CA: Cholic acid; DCA: Deoxycholic acid; CDCA: Chenodeoxycholic acid.
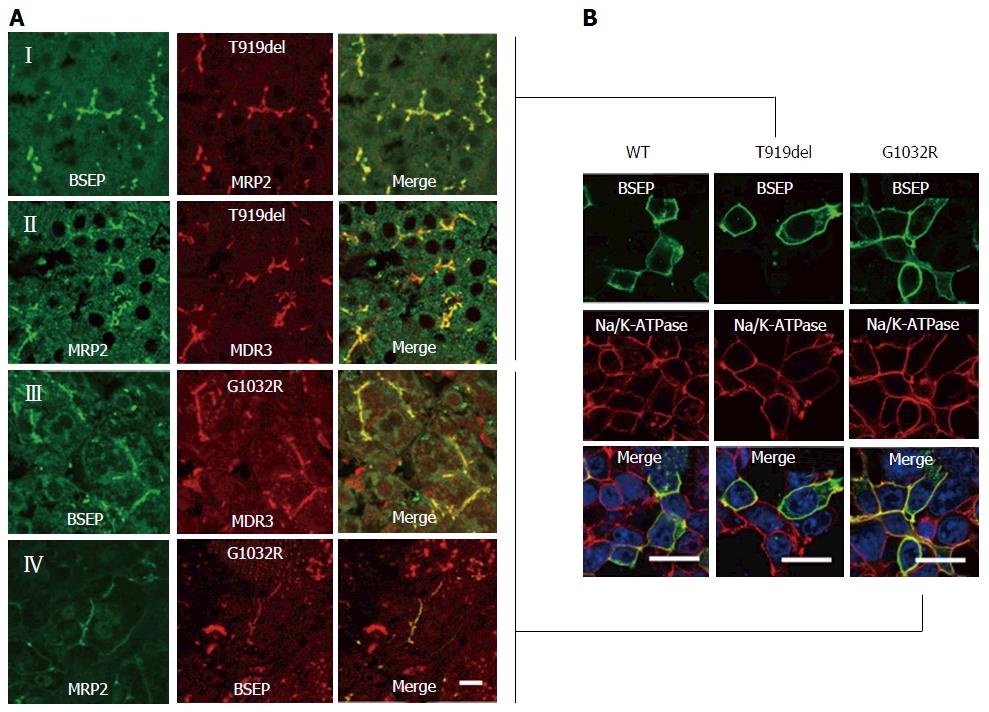
Figure 2 Immunofluorescence studies.
A: Liver biopsy from the female patient possessing the BSEP-T919del mutation. (I) Immunoreactivity of BSEP (K24-antibody) was apparently normal as compared to the bilirubin transporter multidrug resistance- associated protein 2 (MRP2; M2I-4- and M2III-6-antibodies). (II) Immunoreactivity of MDR3 (P3II-26-antibody) showed a typical canalicular pattern similar to MRP2 (EAG5-antibody). Liver biopsy from the male patient (p.G1032R mutation) shows an almost regular BSEP expression (K24-antibody) and MDR3 (III) and MRP2 (IV); B: Wild-type BSEP is largely expressed at the cell membrane of human embryonic kidney (HEK293) cells. Likewise, both BSEP mutants, with deletion of threonine at position 919 (p.T919del) and with arginine instead of glycine at amino acid position 1032 (p.G1032R), are localized at the plasma membrane (Bars = 5 μm). BSEP: Bile salt export pump.
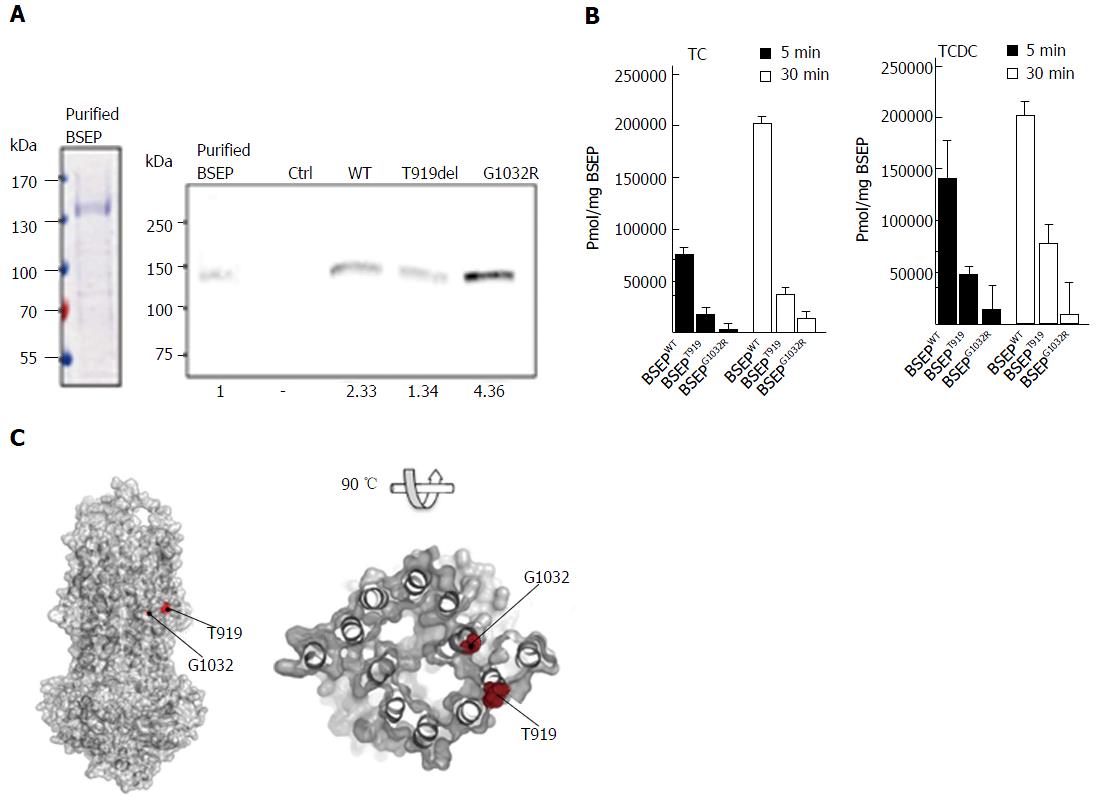
Figure 3 In vitro transport assay of wild-type bile salt export pump, BSEP-T919del and BSEP-G1032R.
A: Left, Coomassie brilliant blue (CBB) stained SDS-PAGE gel of purified Bile Salt Export Pump (BSEP) expressed in Pichia pastoris. 9 μg protein was loaded on the lane. Right, Western blot of purified BSEP and membrane preparations of BSEP-T919del, BSEP-G1032R, BSEP wild-type (WT) and empty control (Ctrl). 38.5 ng of purified BSEP or 15 μL of the membrane preparations were subjected to SDS-PAGE. After detection with the monoclonal antibody, the gel was analyzed densitometrically to calculate the amount of wild-type or mutant BSEP or in the membrane preparations (relative densitometric values below panel); B: [3H]-taurocholate (TC) and [3H]-taurochenodeoxycholate (TCDC) transport by wild-type BSEP, BSEP-T919del, and BSEP-G1032R after 5 (black bars) and 30min (white bars). Experimental details are provided in Materials and Methods. The amount of translocated TC (left) or TCDC (right) per mg of BSEP was determined based on the quantification of wild-type BSEP, BSEP-T919del and BSEP-G1032R in the membrane vesicle preparations. All measurements represent the average with standard deviation of triplicate measurements; C: The homology model of BSEP was generated based on the outward-facing crystal structure of the multidrug transporter Sav18662 from S. aureus (pdb entry: 2HYD). The putative localisation of T919 at the outer surface of the transporter molecule and of G1032 close to the potential translocation pore of BSEP is highlighted by red spheres.Pmol/mg BSEP. BSEP: Bile salt export pump.
- Citation: Ellinger P, Stindt J, Dröge C, Sattler K, Stross C, Kluge S, Herebian D, Smits SHJ, Burdelski M, Schulz-Jürgensen S, Ballauff A, Schulte am Esch J, Mayatepek E, Häussinger D, Kubitz R, Schmitt L. Partial external biliary diversion in bile salt export pump deficiency: Association between outcome and mutation. World J Gastroenterol 2017; 23(29): 5295-5303
- URL: https://www.wjgnet.com/1007-9327/full/v23/i29/5295.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i29.5295