Copyright
©The Author(s) 2017.
World J Gastroenterol. Jul 28, 2017; 23(28): 5167-5178
Published online Jul 28, 2017. doi: 10.3748/wjg.v23.i28.5167
Published online Jul 28, 2017. doi: 10.3748/wjg.v23.i28.5167
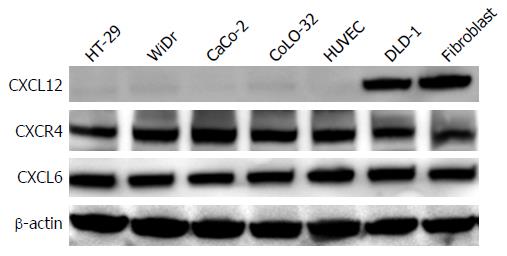
Figure 1 Expression levels of stromal cell-derived factor-1, CXC chemokine receptor 4 and granulocyte chemotactic protein-2 in colon cancer cell lines and stromal cells.
The protein expression levels of CXCL2, CXCR4 and CXCL6 in colon cancer cell lines and stromal cells were determined in whole-cell lysates by western blotting analysis. Thirty micrograms of total cell lysate were subjected to 10% SDS-PAGE and transferred to a polyvinylidene difluoride membrane. The membrane was probed with antibodies to CXCL12, CXCR4 and CXCL6. β-actin was used as a loading control. CXCL6: Granulocyte chemotactic protein-2; CXCL12: Stromal cell-derived factor-1; CXCR4: CXC chemokine receptor 4.
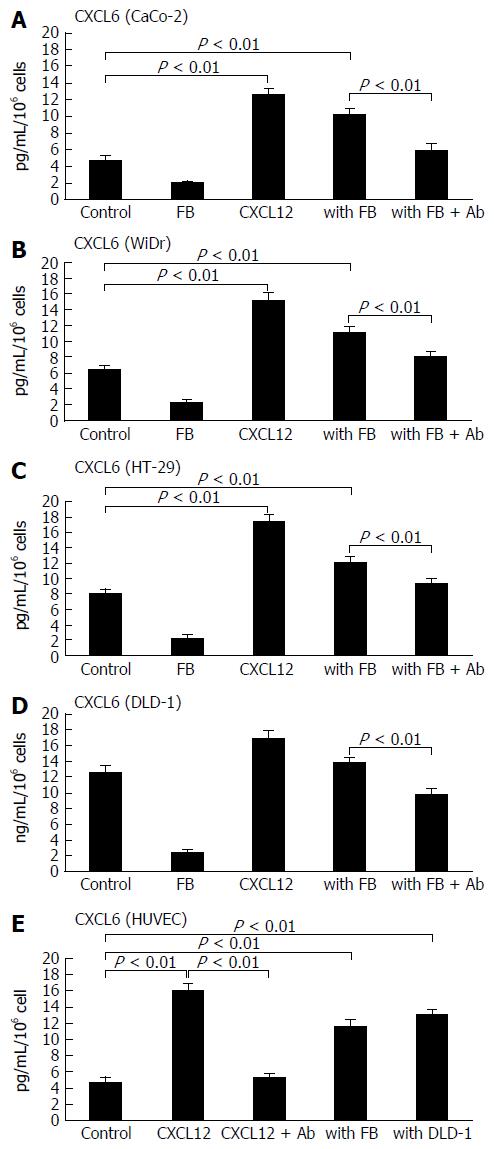
Figure 2 Enhancement of secreted granulocyte chemotactic protein-2 levels in colon cancer cell lines and stromal cells by recombinant stromal cell-derived factor-1 and co-culture with fibroblasts.
The alteration of CXCL6 secretion from colon cancer cell lines [CaCo-2 (A), WiDr (B), HT-29 (C) and DLD-1 (D)] by recombinant CXCL12 stimulation or co-culture with fibroblasts (FB) were determined by enzyme-linked immunosorbent assay in cell culture medium. Meanwhile, colon cancer cells were treated with anti-CXCL12 antibody (Ab) for 2 h, and the concentration of CXCL6 was measured by ELISA in supernatants from colon cancer cells. Effect on secretion of CXCL6 from HUVECs stimulated by recombinant CXCL12 in co-culture system with fibroblasts and the colon cancer cells DLD-1 are shown (E). The experimental detail is described in the “Materials and Methods” section. Control: colon cancer cells only; FB: fibroblasts only; CXCL12: treated with recombinant CXCL12; with FB: colon cancer cells co-cultured with fibroblasts; with FB + Ab: colon cancer cells co-cultured with fibroblasts and pre-treated with anti-CXCL12 Ab. The values are expressed as mean ± SD. Ab: Antibody; CXCL6: Granulocyte chemotactic protein-2; CXCL12: Stromal cell-derived factor-1; HUVEC: Human umbilical vein endothelial cell.
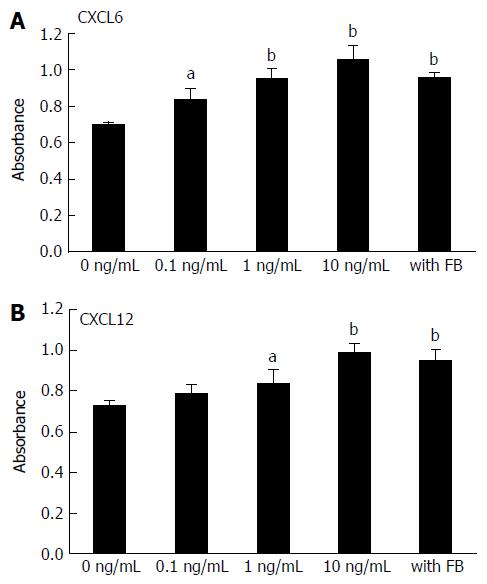
Figure 3 Effect of stromal cell-derived factor-1, granulocyte chemotactic protein-2 and conditioned medium from fibroblasts on human umbilical vein endothelial cell proliferation.
HUVECs were cultured in medium containing different concentrations of CXCL6 (A), CXCL12 (B) and conditioned medium from fibroblasts. After 72 h of incubation, HUVEC proliferation was assessed using premixed WST-1 cell proliferation assay (column mean absorbance reading; Bars = SD). Multiple comparisons were performed by one-way ANOVA followed by the SNK test; aP < 0.05, bP < 0.01. CXCL6: Granulocyte chemotactic protein-2; CXCL12: Stromal cell-derived factor-1; HUVEC: Human umbilical vein endothelial cell.
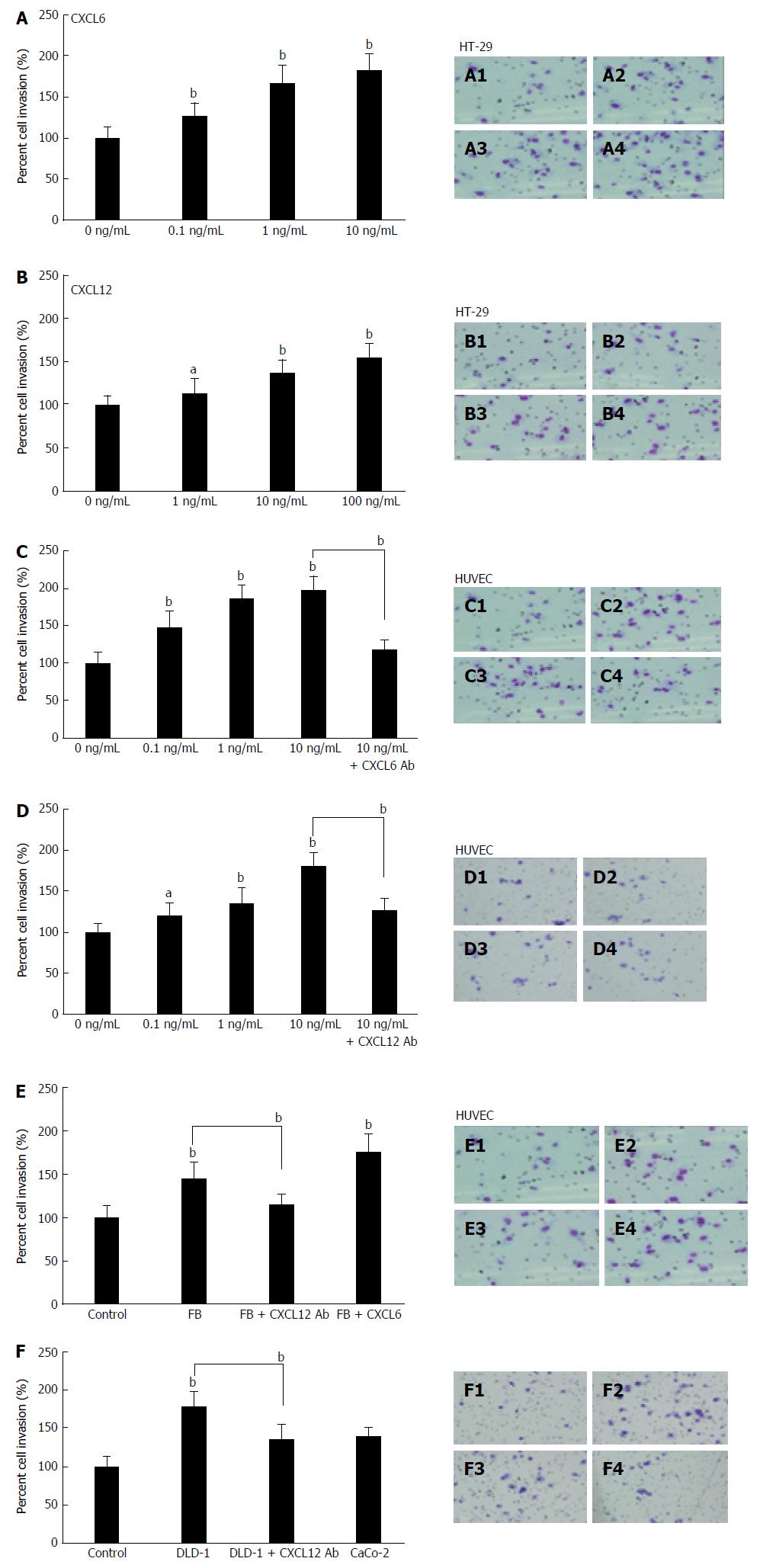
Figure 4 Effect of granulocyte chemotactic protein-2, stromal cell-derived factor-1 and co-culture with fibroblasts or DLD-1 on colon cancer cell and human umbilical vein endothelial cell invasiveness.
The influence of different concentrations of CXCL6 (A), CXCL12 (B) or co-culture with fibroblasts on colon cancer cell invasiveness was measured by the BD Bio-Coat Matrigel invasion assay system (BD Biosciences). HT-29 (A and B) cells and HUVECs (C and D) were pre-treated with different concentrations of CXCL6 and CXCL12, and co-culture with fibroblasts (E) or DLD-1 (F), or pre-treated with or without anti CXCL6 or CXCL12 antibody, and following a 24-h incubation. The invading cells were fixed and stained with Diff-Quick stain. Invading cells were counted in five random microscopic fields (× 200). The experiment detail is described in the “Material and Methods” section. Multiple comparisons were performed by one-way ANOVA followed by the SNK test; aP < 0.05, bP < 0.01. A1: HT-29 cells only; A2: 0.1 ng/mL of CXCL6; A3: 1 ng/mL of CXCL6; A4: 10 ng/mL of CXCL6. B1: HT-29 cells only; B2: 0.1 ng/mL of CXCL12; B3: 1 ng/mL of CXCL12; B4: 10 ng/mL of CXCL12; C1: HUVECs only; C2: 1 ng/mL of CXCL6; C3: 10 ng/mL of CXCL6; C4: 10 ng/mL of CXCL6 treated with 10 μg/mL CXCL6 Ab. D1: HUVECs only; D2: 1 ng/mL of CXCL12; D3: 10 ng/mL of CXCL12; D4: 10 ng/mL of CXCL12 treated with 10 μg/mL CXCL12 Ab. E1: HUVECs only; E2: HUVECs co-culture with fibroblasts; E3: Co-culture with fibroblasts + 10 μg/mL CXCL12 Ab; E4: Co-culture with fibroblasts + 10 ng/mL CXCL6. F1: HUVECs only; F2: HUVECs co-culture with DLD-1 cells; F3: Co-culture with DLD-1 cells + 10 μg/mL CXCL12 Ab; F4: Co-culture with CaCo-2 cells. Ab: Antibody; CXCL6: Granulocyte chemotactic protein-2; CXCL12: Stromal cell-derived factor-1; HUVEC: Human umbilical vein endothelial cell.
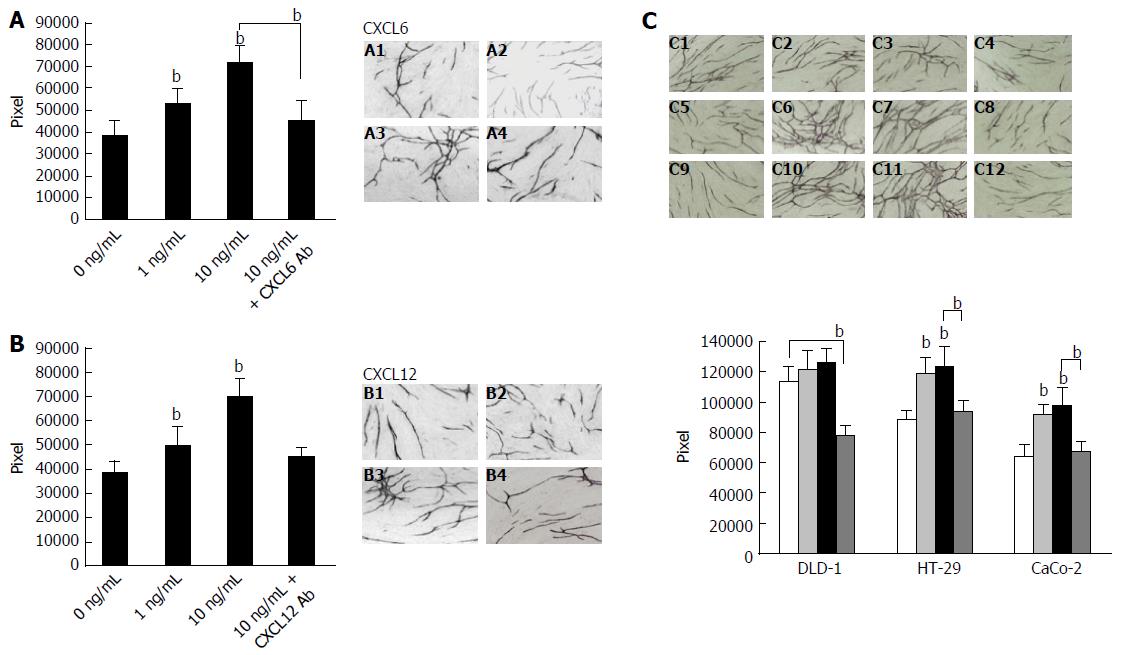
Figure 5 Effect of granulocyte chemotactic protein-2, stromal cell-derived factor-1 and co-culture with colon cancer cells on angiogenesis.
The treatment of CXCL6 (A) and CXCL12 (B) influence HUVEC tube formation. After incubation of the HUVEC/fibroblast co-culture system in the presence or absence of CXCL6 or anti-CXCL12 Ab, then co-culture for 7 d, the HUVEC/fibroblast co-culture system was stained with anti-CD31 antibody. Tube formation area was measured quantitatively using an image analyzer. A1: Control; A2: 1 ng/mL CXCL6; A3: 10 ng/mL CXCL6; A4: 10 ng/mL CXCL6 + 10 μg/mL of CXCL6 Ab. B1: Control; B2: 1 ng/mL CXCL12; B3: 10 ng/mL CXCL12; B4: 10 ng/mL CXCL12 + 10 μg/mL of CXCL12 Ab. Effect of colon cancer cells (DLD-1, HT-29 or CaCo-2) on HUVEC tube formation is shown (C). Angiogenesis assay by HUVEC/fibroblast co-culture with DLD-1, HT-29 or CaCo-2 cells was conducted using the double-chamber method. Detection of tube formation by HUVECs was described in the “Material and Methods” section. C1: Co-culture with DLD-1; C2: Co-culture with DLD-1 + 10 ng/mL of CXCL6; C3: Co-culture with DLD-1 + 10 ng/mL of CXCL12; C4: Co-culture with DLD-1 + 10 μg/mL of CXCL12 Ab; C5: Co-culture with HT-29 cells; C6: Co-culture with HT-29 cells pre-treated with 10 ng/mL CXCL6; C7: Co-culture with HT-29 cells pre-treated with 10 ng/mL CXCL12; C8: Co-culture with HT-29 + 10 μg/mL of CXCL12 Ab; C9: Co-culture with CaCo-2 cells; C10: Co-culture with CaCo-2 cells pre-treated with 10 ng/mL CXCL6; C11: Co-culture with CaCo-2 cells pre-treated with 10 ng/mL CXCL12; C12: Co-culture with CaCo-2 cells pre-treated with 10 μg/mL anti-CXCL12 antibody. Columns, mean pixels of HUVEC tube formation area; Bars = SD. Multiple comparisons were performed by one-way ANOVA followed by the SNK test; bP < 0.01 vs control. Ab: Antibody; CXCL6: Granulocyte chemotactic protein-2; CXCL12: Stromal cell-derived factor-1; HUVEC: Human umbilical vein endothelial cell.
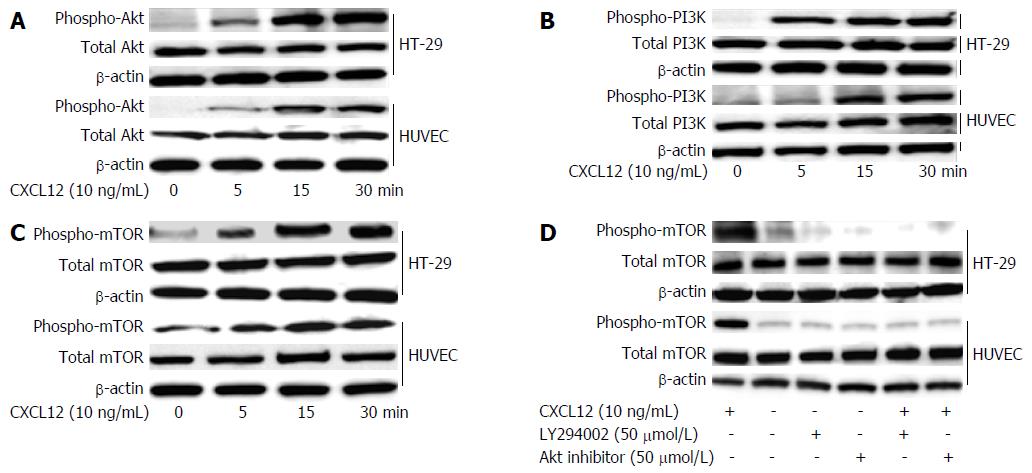
Figure 6 Stromal cell-derived factor-1-induced phosphorylation of PI3K/Akt/mTOR signaling in colon cancer cell lines and stromal cells.
HT-29 cells and HUVECs were treated with 10 ng/mL of CXCL12 cultured for 5, 10 and 30 min. The cells were collected and lysed by lysis buffer. Aliquots of 30 μg of lysed protein were subjected to immunoblotting with a phospho-Akt (A), phospho-PI3K (B) and phosphor-mTOR (C) Abs. Detection of total Akt, PI3K or mTOR levels aided in loading control. HT-29 cells or HUVECs, after being pre-treated with 50 μmol/L Akt inhibitor and 50 μmol/L LY294002 for 1 h, were incubated with 10 ng/mL CXCL12 for 1 h. Results of immunoblotting using the mTOR Ab is shown (D). Detection of total mTOR levels served as loading control. Ab: Antibody; CXCL6: Granulocyte chemotactic protein-2; CXCL12: Stromal cell-derived factor-1; HUVEC: Human umbilical vein endothelial cell.
- Citation: Ma JC, Sun XW, Su H, Chen Q, Guo TK, Li Y, Chen XC, Guo J, Gong ZQ, Zhao XD, Qi JB. Fibroblast-derived CXCL12/SDF-1α promotes CXCL6 secretion and co-operatively enhances metastatic potential through the PI3K/Akt/mTOR pathway in colon cancer. World J Gastroenterol 2017; 23(28): 5167-5178
- URL: https://www.wjgnet.com/1007-9327/full/v23/i28/5167.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i28.5167