Copyright
©The Author(s) 2016.
World J Gastroenterol. Jan 28, 2016; 22(4): 1433-1448
Published online Jan 28, 2016. doi: 10.3748/wjg.v22.i4.1433
Published online Jan 28, 2016. doi: 10.3748/wjg.v22.i4.1433
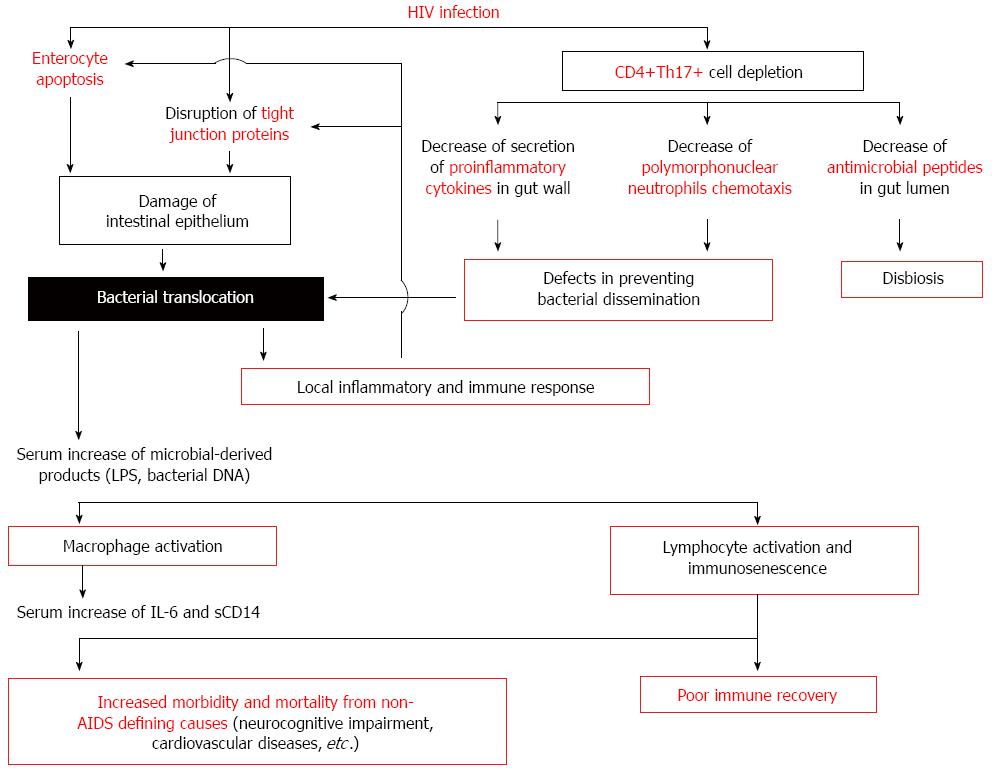
Figure 1 Effect of bacterial translocation on innate and acquired immune activation in human immunodeficiency virus-infected patients.
In the gastrointestinal tract, human immunodeficiency virus (HIV) infection induces a Th17 CD4+ T helper cells depletion and a damage of intestinal epithelium as a consequence of the HIV exposure itself or by immune-induced enterocyte damage. The consequence of the alteration in gut barrier is the bacterial translocation. Increased plasma concentrations of several markers indicative of pathological bacterial translocation [lipopolysaccharide (LPS), bacterial DNA] have been detected in these patients. These molecules induce a monocyte and lymphocyte activation implicated in the increased morbidity and mortality from non-acquired immunodeficiency syndrome (AIDS) defining causes (neurocognitive impairment, cardiovascular diseases, etc.) and in the poor immune recovery. IL: Interleukin; sCD14: Soluble CD14.
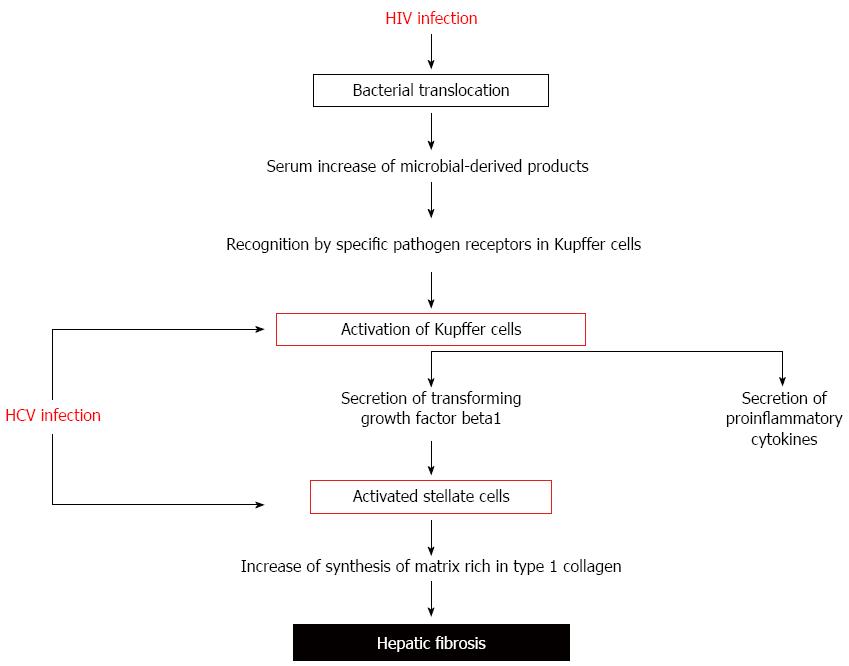
Figure 2 Increased fibrogenesis rate in human immunodeficiency virus-hepatitis C virus coinfected patients.
In human immunodeficiency virus (HIV)-hepatitis C virus (HCV) coinfection, translocated bacterial products contribute to liver disease progression by binding to specific pathogen recognition receptors in Kupffer cells. Activated Kupffer cells secrete proinflammatory cytokines and transforming growth factor β1. Activated stellate cells, stimulated by transforming growth factor β1, produce a matrix rich in type 1 collagen, increasing the effect of HCV on hepatic fibrosis.
- Citation: Márquez M, Fernández Gutiérrez del Álamo C, Girón-González JA. Gut epithelial barrier dysfunction in human immunodeficiency virus-hepatitis C virus coinfected patients: Influence on innate and acquired immunity. World J Gastroenterol 2016; 22(4): 1433-1448
- URL: https://www.wjgnet.com/1007-9327/full/v22/i4/1433.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i4.1433