Copyright
©The Author(s) 2016.
World J Gastroenterol. Jan 7, 2016; 22(1): 262-274
Published online Jan 7, 2016. doi: 10.3748/wjg.v22.i1.262
Published online Jan 7, 2016. doi: 10.3748/wjg.v22.i1.262
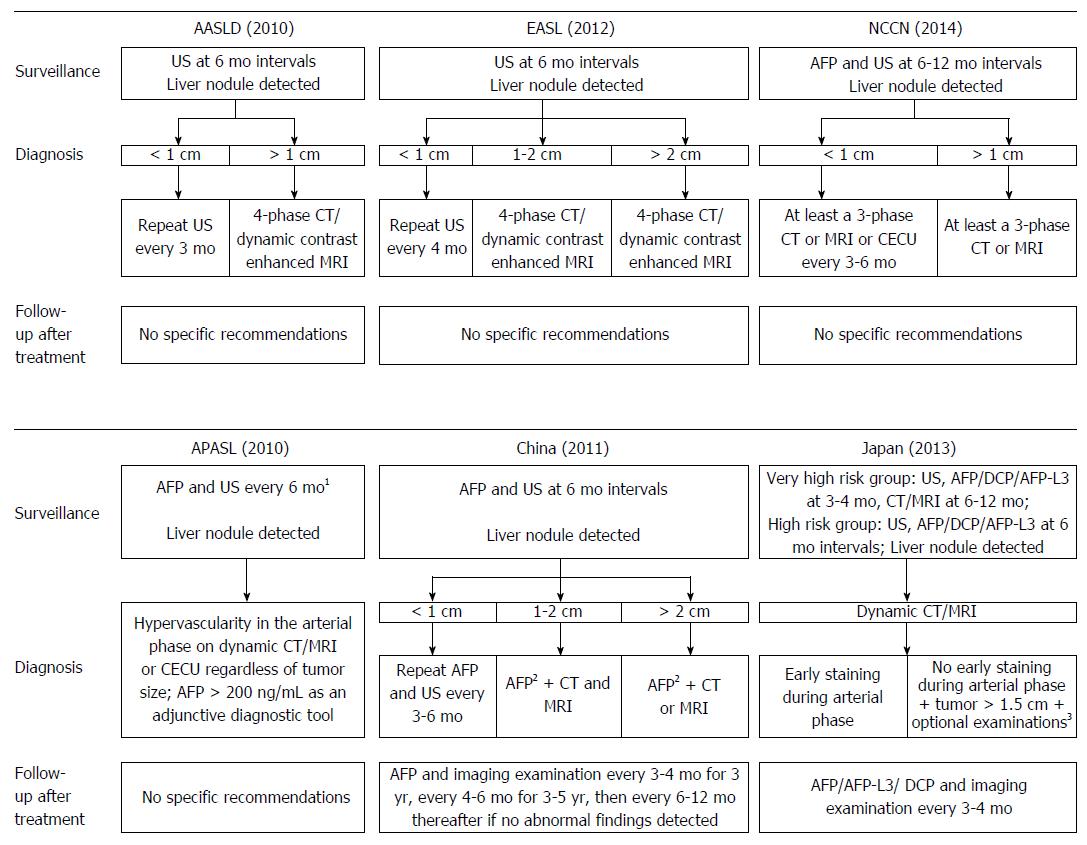
Figure 1 The clinical utility of biomarkers according to typical hepatocellular carcinoma guidelines worldwide.
A: Typical hepatocellular carcinoma (HCC) guidelines in Western countries; B: Typical HCC guidelines in Asian countries. 1Alpha-fetoprotein (AFP) alone is not recommended for diagnosis of HCC; the measurement of both AFP and des-γ-carboxyprothrombin (DCP) provides a higher level of sensitivity without decreasing specificity; 2AFP ≥ 400 ng/mL over 1 mo or AFP ≥ 200 ng/mL over 2 mo; 3Optional examinations include computed tomography (CT)-angiography, liver-specific contrast-enhanced magnetic resonance imaging (MRI), contrast ultrasound (US), or liver tumor biopsy.
- Citation: Song PP, Xia JF, Inagaki Y, Hasegawa K, Sakamoto Y, Kokudo N, Tang W. Controversies regarding and perspectives on clinical utility of biomarkers in hepatocellular carcinoma. World J Gastroenterol 2016; 22(1): 262-274
- URL: https://www.wjgnet.com/1007-9327/full/v22/i1/262.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i1.262