Copyright
©The Author(s) 2015.
World J Gastroenterol. Nov 21, 2015; 21(43): 12249-12260
Published online Nov 21, 2015. doi: 10.3748/wjg.v21.i43.12249
Published online Nov 21, 2015. doi: 10.3748/wjg.v21.i43.12249
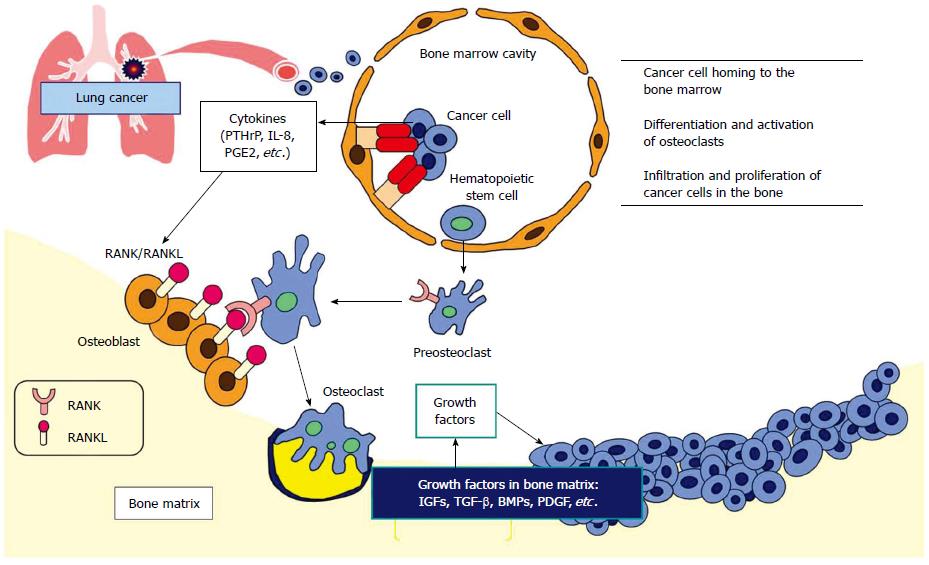
Figure 1 Molecular mechanism of osteolytic bone metastasis.
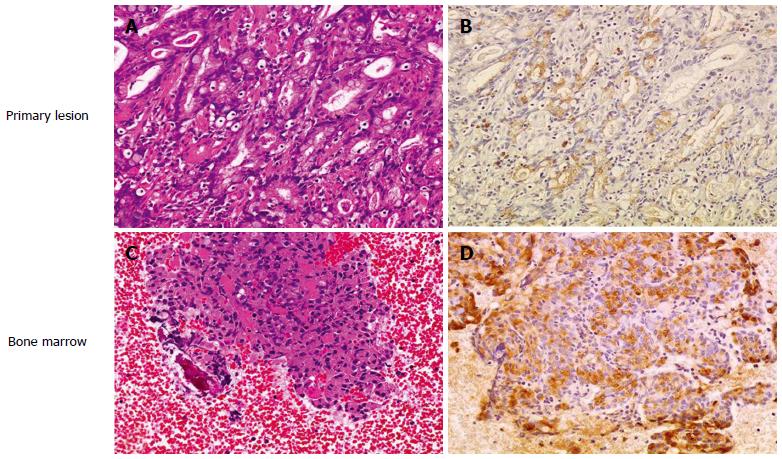
Figure 2 RANKL expression in disseminated carcinomatosis of the bone marrow associated with gastric cancer.
Representative immunohistochemistry for RANKL in gastric cancer demonstrating disseminated carcinomatosis of the bone marrow. A: Hematoxylin and eosin staining of gastric cancer shows moderately differentiated adenocarcinoma (magnification × 20); B: Immunohistochemistry for RANKL in a serial section of the same specimen in (A). RANKL shows positive staining predominantly in the cytoplasm and plasma membrane of moderately differentiated adenocarcinoma cells (magnification × 20); C: Hematoxylin and eosin staining of a bone marrow aspiration smear shows infiltration of atypical epithelial cells, indicating metastasis from known gastric cancer (magnification × 20); D: Immunohistochemistry for RANKL in a serial section of the same specimen in (C). RANKL shows positive staining predominantly in the cytoplasm and plasma membrane of metastatic gastric cancer cells as is seen in the primary lesion (B) (magnification × 20). Adapted from Kusumoto et al[9].
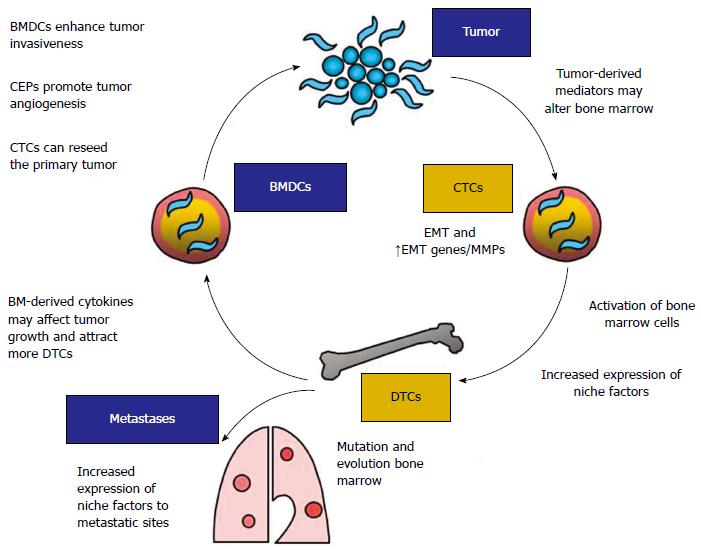
Figure 3 Association of disseminated tumor cells in the bone marrow with cancer metastasis.
DTCs: Disseminated tumor cells; CTCs: Circulating tumor cells; CEPs: Circulating endothelial progenitors; MMP: Matrix metalloproteinase; BM: Bone marrow; BMDCs: BM-derived cells; EMT: Epithelial-mesenchymal transition. Adapted from Aft et al[21].
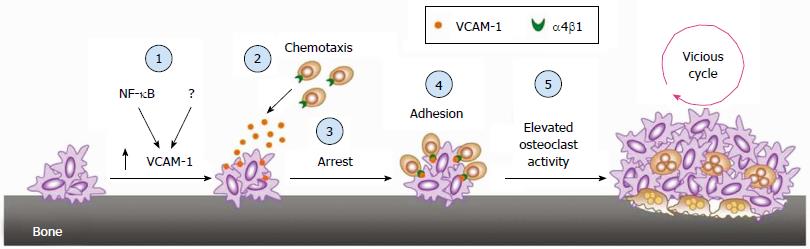
Figure 4 Reactivation of disseminated tumor cells in the bone marrow: Transition from dormant micrometastasis (i.
e., DTCs) to micrometastasis. Incidental activation of VCAM-1, a process possibly dependent on NF-κB signaling and other unidentified mechanisms (1), in micrometastasis arrests α4b1-positive osteoclast progenitors through paracrine chemotaxis (2) and adhesion (3), this leads to a localized increase of te osteoclast progenitor population and increased mature osteoclast activity (4), activated osteoclasts resorb the bone and instigate the vicious cycle of bone metastasis (5). DTCs: Disseminated tumor cells. Adapted from Lu et al[22].
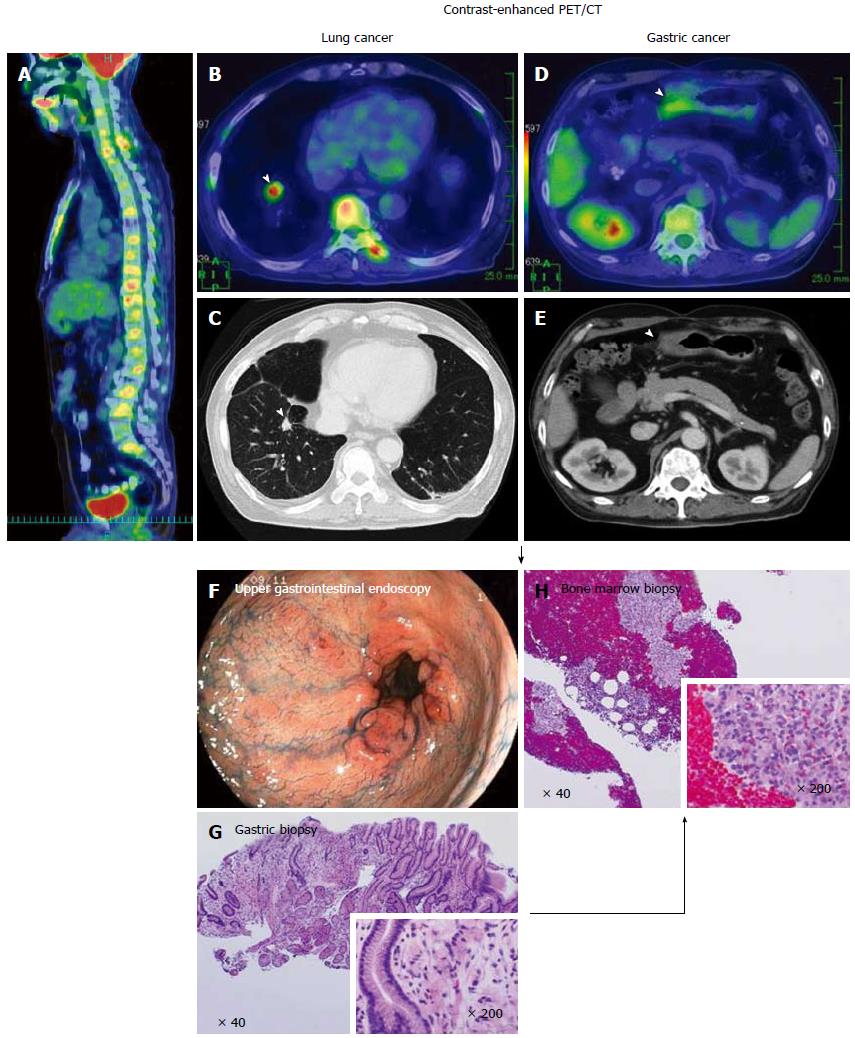
Figure 5 A case of synchronous disseminated carcinomatosis of the bone marrow associated with gastric cancer in a 60-year-old man: Findings of positron emission tomography/computed tomography imaging, endoscopic examination, and bone marrow biopsy.
History: The patient visited a local doctor in August 2007 with complaints of left chest and back pain. CT and magnetic resonance imaging revealed a “lung tumor” and “bone metastasis (thoracic vertebrae)”; therefore, the patient was referred to the Shikoku Cancer Center. Laboratory findings: Marked elevation of serum ALP (2594 U/L), CRP (34.74 mg/dL), and mild elevation of serum LDH (342 U/L) were observed with hematological disorder (DIC and elevated WBC [possible leukoerythroblastosis]). Tumor marker (CA19-9 162 U/mL) and bone metabolic markers (1CTP 8.9 ng/mL; BAP 258 μg/L) were elevated. These findings were suggestive of recurrence of gastric cancer, perhaps disseminated carcinomatosis of the bone marrow. PET/CT imaging: FDG accumulation was observed in all spinal vertebrae and the sternum in the sagittal view of the PET/CT fusion image (A). In the transaxial views of CT and PET/CT fusion images, a tumor mass with FDG accumulation was observed in the right lung (arrowheads; B, C) and thickening of the wall with FDG accumulation was observed in the gastric antrum (arrowheads; D, E). These findings on PET/CT suggested the presence of lung cancer together with gastric cancer. Endoscopic examination: Multiple erosions were observed in the gastric antrum/pyloric ring (F). In the tissue specimen obtained from the erosions, proliferation of signet-ring cell carcinoma was observed (G). Bone marrow biopsy: In order to determine the origin of the bone lesions, bone marrow biopsy was performed. Signet-ring cell carcinoma characterized by clear abundant cytoplasm and eccentrically positioned nuclei was found in the hematopoietic bone marrow (H). This histologic finding suggests the bone lesions originated from the gastric cancer. PET: Positron emission tomography; CT: Computed tomography.
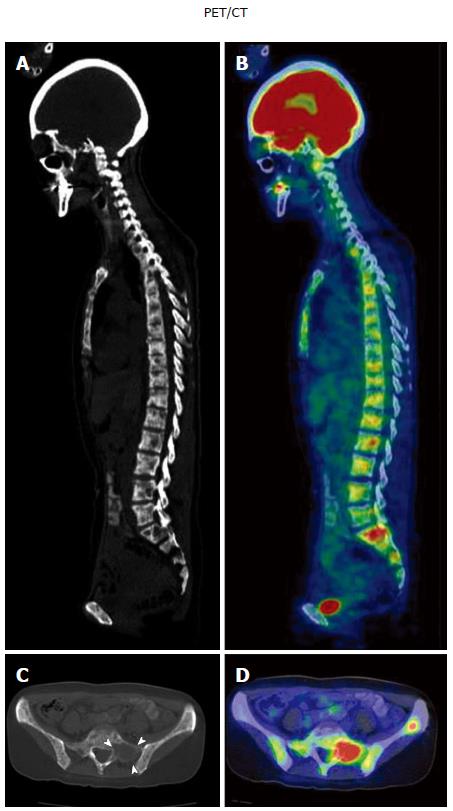
Figure 6 A case of metachronous disseminated carcinomatosis of the bone marrow associated with gastric cancer in a 47-year-old woman: Laboratory findings and positron emission tomography/computed tomography imaging.
History: The patient underwent gastric cancer surgery at 36 years of age (histological diagnosis, poorly differentiated adenocarcinoma < signet-ring cell carcinoma), followed by chemotherapy with oral 5-FU (postoperative adjuvant therapy) for 3 years. Eleven years postoperatively, she visited a local orthopedist with a complaint of low back pain. Elevated serum ALP and multiple bone metastases were found on bone scintigraphy, and she was referred to the Shikoku Cancer Center. Laboratory findings: Marked elevation of serum ALP (11740 IU/L) and mild elevation of serum LDH (435 IU/L) were observed with hematological disorders (i.e., DIC and anemia: Hb 6.9 g/dL). Tumor markers (CEA 241 ng/mL; CA19-9 212 U/mL) and bone metabolic markers (1CTP 17.7 ng/mL; Urine NTx > 300 nmol BCE/L; BAP 1260 μg/L) were elevated. These findings were suggestive of recurrence of the gastric cancer in the bone (i.e., disseminated carcinomatosis of the bone marrow). PET/CT imaging: Osteolytic changes with FDG accumulation were observed in most vertebrae. A, B: Sagittal views of CT (A) and PET/CT fusion images (B); C, D: Transaxial views of the sacrum (S1) on CT (C; arrowheads, osteolytic change) and PET/CT fusion image (D). PET: Positron emission tomography; CT: Computed tomography.
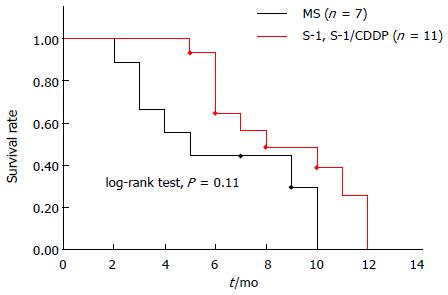
Figure 7 Kaplan-Meier survival curves of disseminated carcinomatosis of the bone marrow associated with gastric cancer according to chemotherapy regimen.
Among 28 cases of disseminated carcinomatosis of the bone marrow associated with gastric cancer reported in Japan during 2003-2013, 27 cases were treated with chemotherapy. In these 27 cases, information about survival time was available for 18 cases. Using these data, Kaplan-Meier survival curves were calculated according to the chemotherapy regimen.
- Citation: Iguchi H. Recent aspects for disseminated carcinomatosis of the bone marrow associated with gastric cancer: What has been done for the past, and what will be needed in future? World J Gastroenterol 2015; 21(43): 12249-12260
- URL: https://www.wjgnet.com/1007-9327/full/v21/i43/12249.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i43.12249