Copyright
©The Author(s) 2015.
World J Gastroenterol. Oct 7, 2015; 21(37): 10553-10562
Published online Oct 7, 2015. doi: 10.3748/wjg.v21.i37.10553
Published online Oct 7, 2015. doi: 10.3748/wjg.v21.i37.10553
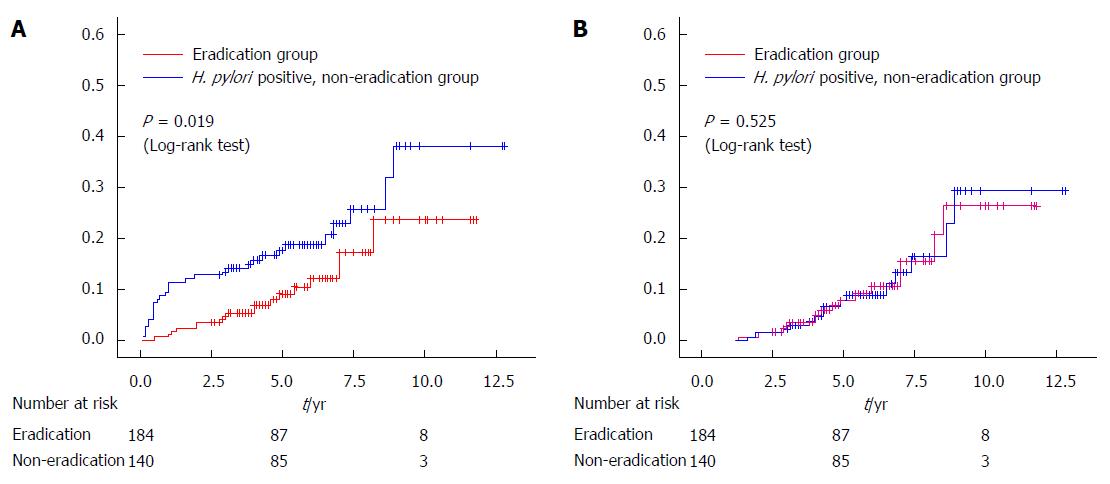
Figure 1 Kaplan-Meier analysis of the cumulative incidence rate of metachronous cancer in patients with and without Helicobacter pylori eradication therapy after endoscopic resection of early gastric cancer (adapted from our institute data[26]).
A: When second lesions detected within 1 year after endoscopic resection (ER) were handled as metachronous cancers, the detection rate in the eradication group was reduced significantly (P = 0.019, Log-rank test) compared with the non-eradication group; B: In contrast, when second lesions were defined as overlooked synchronous cancers and excluded from the metachronous cancer group, there was no significant difference between the two groups.
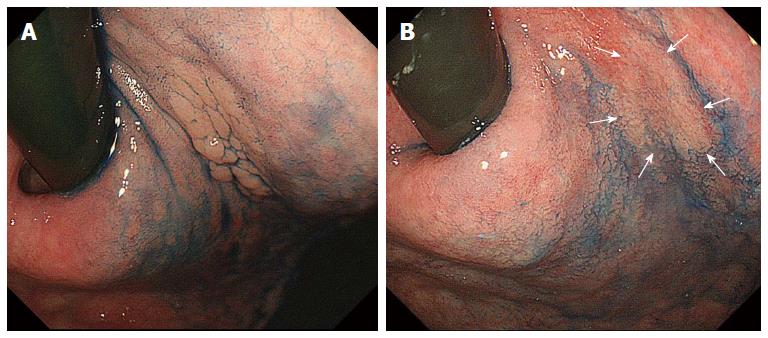
Figure 2 Gastric adenoma detected before 4 years with a granular appearance (A) and this lesion became flat and the granules disappeared after successful Helicobacter pylori eradication therpy (B).
It was difficult to identify the border (arrows) by chromoendoscopy. After endoscopic submucosal dissection, the histological diagnosis was adenocarcinoma[46].
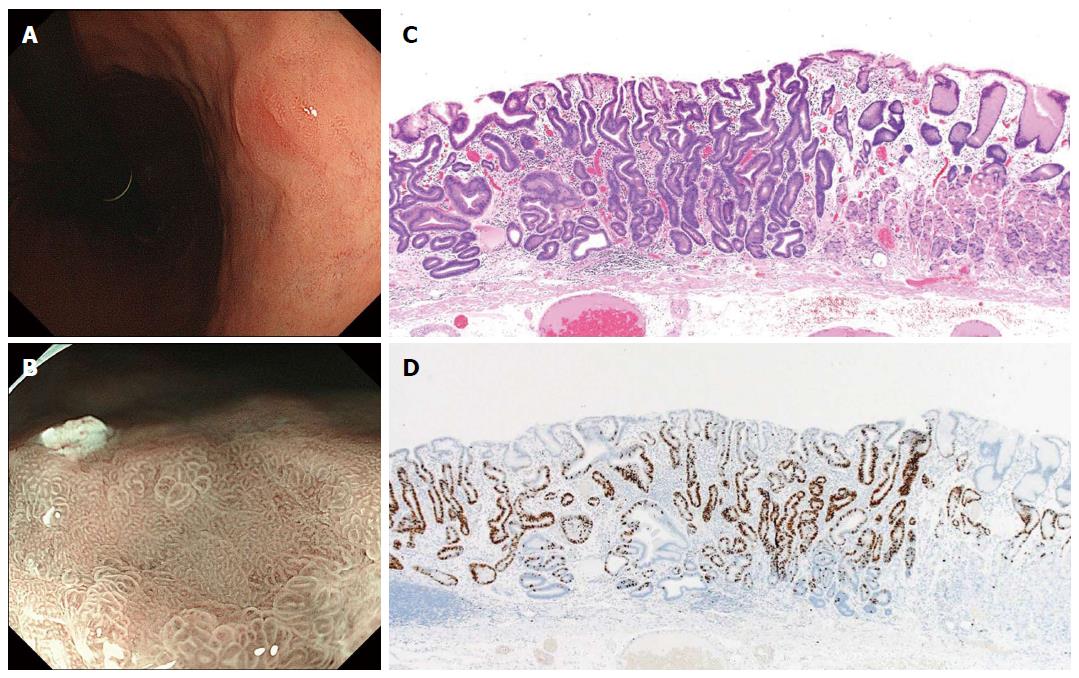
Figure 3 Differentiated-type early gastric cancer detected after successful Helicobacter pylori eradication.
A: This endoscopic finding made qualitative cancer diagnosis difficult by conventional endoscopy; B: A “gastritis-like” appearance under narrow-band imaging with magnified endoscopy was demonstrated by a microsurface structure comprised of mixed pits and papillae, resembling the surrounding non-cancerous mucosa; C: Well-differentiated tubular adenocarcinoma with low-grade atypia (hematoxylin and eosin staining); D: Ki-67 positive cells localized in the middle-to-lower portion of the mucosa, indicating cytological maturation at the luminal surface layer of the cancer[47].
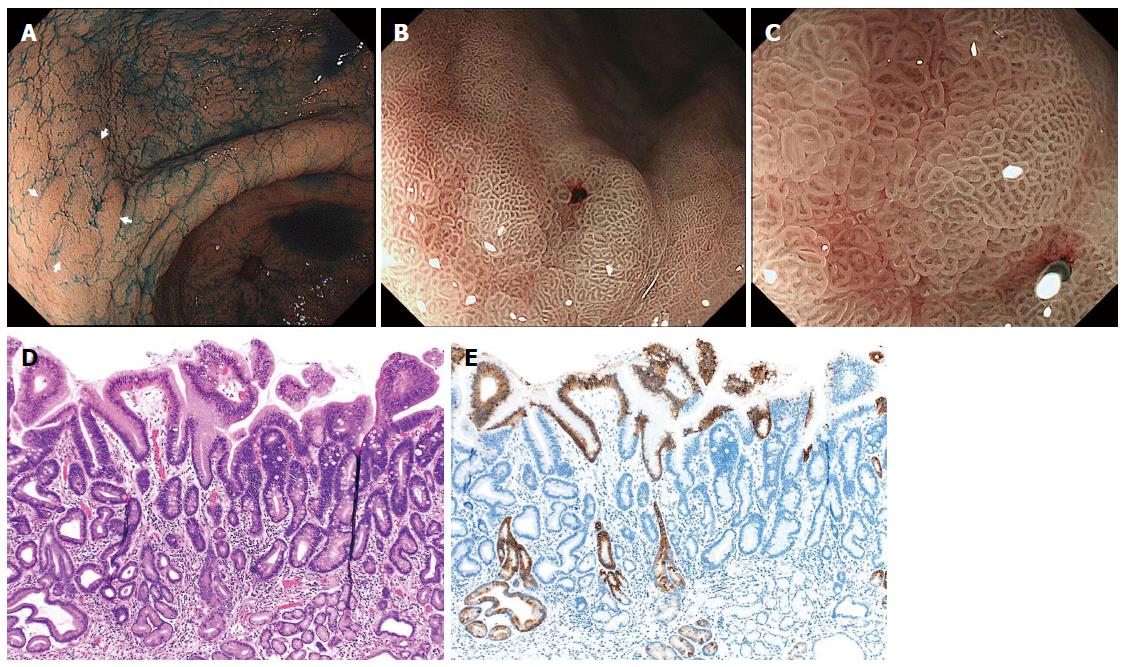
Figure 4 Differentiated-type early gastric cancer detected after successful Helicobacter pylori eradication.
A: Chromoendoscopy revealed a depressed-type lesion in the anterior wall of the antrum; B, C: Narrow-band imaging with magnified endoscopy shows a “gastritis-like” appearance with a microsurface structure comprising of regular papillae resembling with unclear demarcation between the cancer and surrounding gastritis mucosa; D: Well-differentiated tubular adenocarcinoma with low-grade atypia (hematoxylin and eosin staining); E: MUC5AC immunohistochemical staining demonstrates that superficial non-neoplastic epithelium is interspersed among and above the cancer tubules[47].
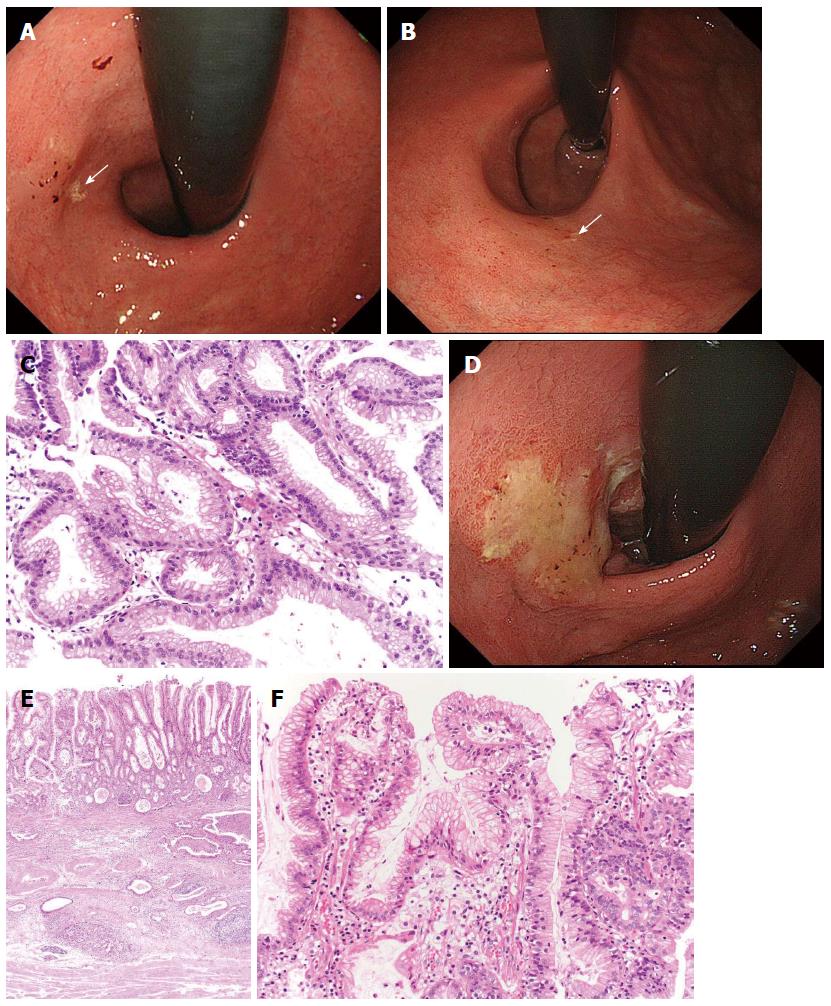
Figure 5 Gastric cancer progressed after eradication therapy.
A: A review of the endoscopic pictures taken 3 years earlier revealed the presence of a discolored and flat elevated lesion adjacent to a xanthoma (arrow) in the cardia; B: This lesion was not clear yet, but the xanthoma had almost disappeared (arrow); C: The biopsy specimen taken from the lesion showed foveolar-type epithelium with mild structural atypia; D: After follow-up for 1 yr, the tumor had become evident and covered by mucus; E: The tumor had invaded into the deep submucosal layer, but the mucosal element was preserved; F: This cancer showed a gastric mucin phenotype with low-grade atypia[26].
-
Citation: Kobayashi M, Sato Y, Terai S. Endoscopic surveillance of gastric cancers after
Helicobacter pylori eradication. World J Gastroenterol 2015; 21(37): 10553-10562 - URL: https://www.wjgnet.com/1007-9327/full/v21/i37/10553.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i37.10553