Copyright
©The Author(s) 2015.
World J Gastroenterol. Jul 28, 2015; 21(28): 8492-8507
Published online Jul 28, 2015. doi: 10.3748/wjg.v21.i28.8492
Published online Jul 28, 2015. doi: 10.3748/wjg.v21.i28.8492
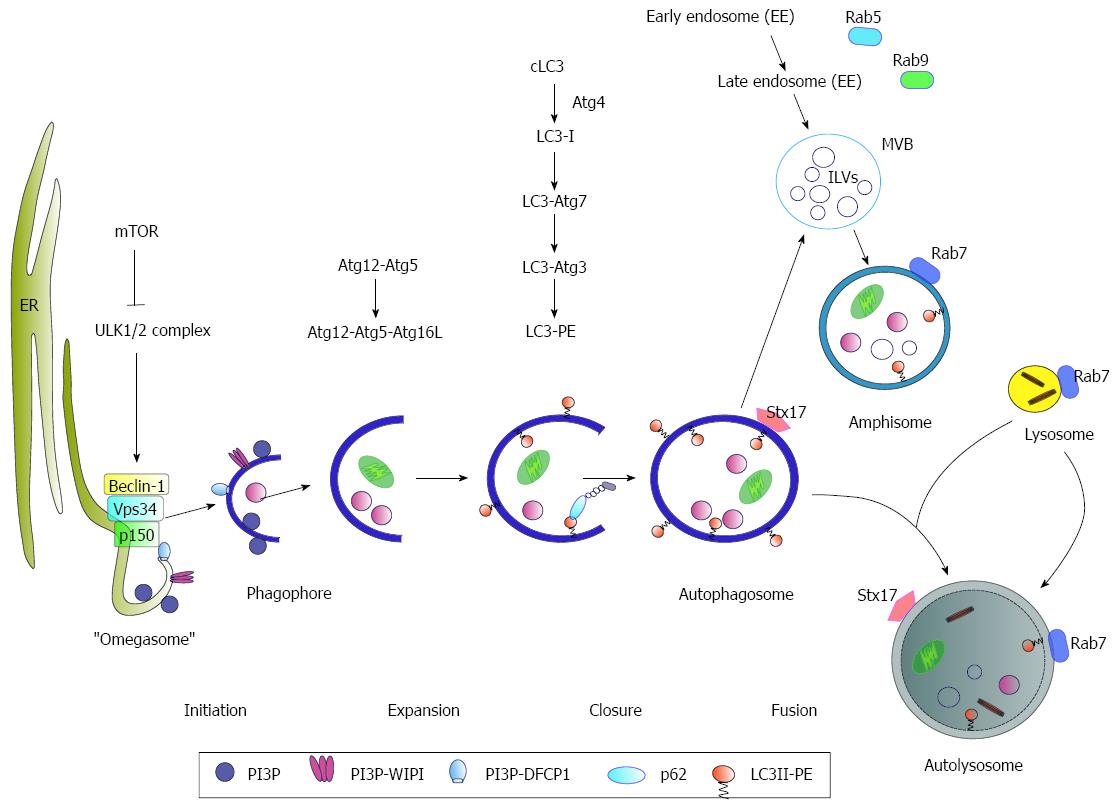
Figure 1 Schematic model of autophagy.
Under nutrient-rich conditions mammalian target of rapamycin (mTOR) represses the ULK1/2 complex. During starvation, mTOR kinase is inhibited resulting in activation of autophagy. As an initial step, Beclin-1 forms a complex with Vps34 and p150 (PI3K complex). The activated ULK1/2 and PI3K complex catalyze the formation of a PI3P (phosphatidylinositol-3-phosphate)-enriched environment. The PI3P further recruits the effector double FYVE-containing protein 1 (DFCP1) and WD-repeat domain phosphoinositide-interacting (WIPI) proteins, leading to phagophore nucleation by formation of an ER-associated Ω-structure (“omegasome”). After the nucleation process the formation of the autophagosome occurs. Here, the Atg5-Atg12-Atg16L complex and LC3 II, conjugated to phosphatidylethanolamine (PE) (LC3-PE), play a crucial role in the elongation and closure of the membrane. The autophagosomes can either directly fuse with lysosomes to form the autolysosome or fuse with late endosomes/multivesicular bodies (MVBs) forming an amphisome prior fusion with the lysosome where their cargo is digested. Rab7-GTPase activity triggers the fusion of the autophagosome with the lysosome. Another factor involved in autophagosome-lysosome fusion is the autophagosomal SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein) syntaxin 17 (Stx17) that can be found on the outer membrane of completed enclosed autophagosomes.
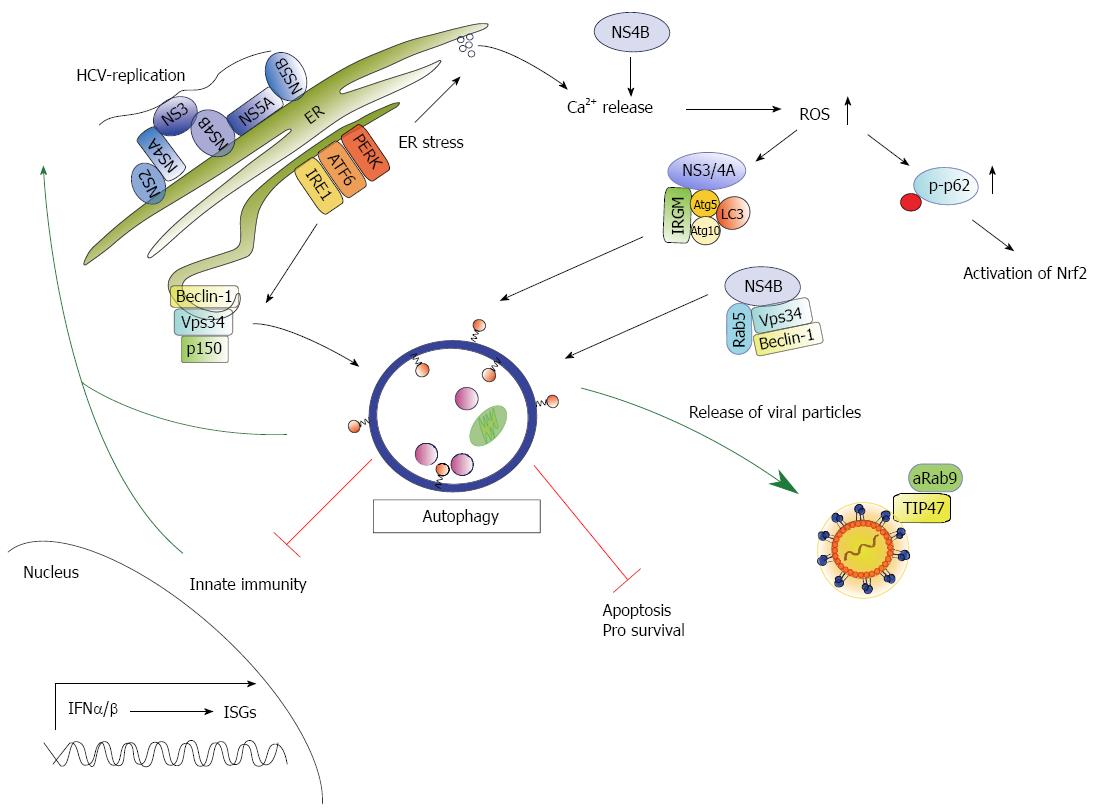
Figure 2 Schematic model of the crosstalk between hepatitis C virus and autophagy.
Hepatitis C virus (HCV) replication occurs in replication complexes that assemble at the ER resulting in ER stress and induction of an unfolded protein response (UPR). The UPR activates three signaling pathways (ATF6, IRE1, PERK) and finally autophagy. In addition the UPR can be triggered by NS4B through activation of the IRE1 and ATF6 pathway as NS4B interferes with Ca2+ homeostasis resulting in elevated reactive oxidant species (ROS). Elevated ROS levels trigger phosphorylation of p62 on Ser351 (p-p62) following subsequent activation of Nrf2. NS4B further forms a complex with Rab5, Vps34 and Beclin-1 stimulating an autophagic response. In addition the human immunity-associated GTPase family M (IRGM) protein interacts with NS3/4A and the autophagosomal protein Atg5, Atg10 and LC3 that trigger autophagy. Activation of autophagy favors HCV replication and plays a crucial role in release of viral particles. Here, the interaction of TIP47 and activated Rab9 (aRab9) to the particles is essential. Moreover, HCV-induced autophagy impairs innate immunity and favors cell survival via inhibition of apoptosis.
- Citation: Ploen D, Hildt E. Hepatitis C virus comes for dinner: How the hepatitis C virus interferes with autophagy. World J Gastroenterol 2015; 21(28): 8492-8507
- URL: https://www.wjgnet.com/1007-9327/full/v21/i28/8492.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i28.8492