Copyright
©The Author(s) 2015.
World J Gastroenterol. May 14, 2015; 21(18): 5473-5481
Published online May 14, 2015. doi: 10.3748/wjg.v21.i18.5473
Published online May 14, 2015. doi: 10.3748/wjg.v21.i18.5473
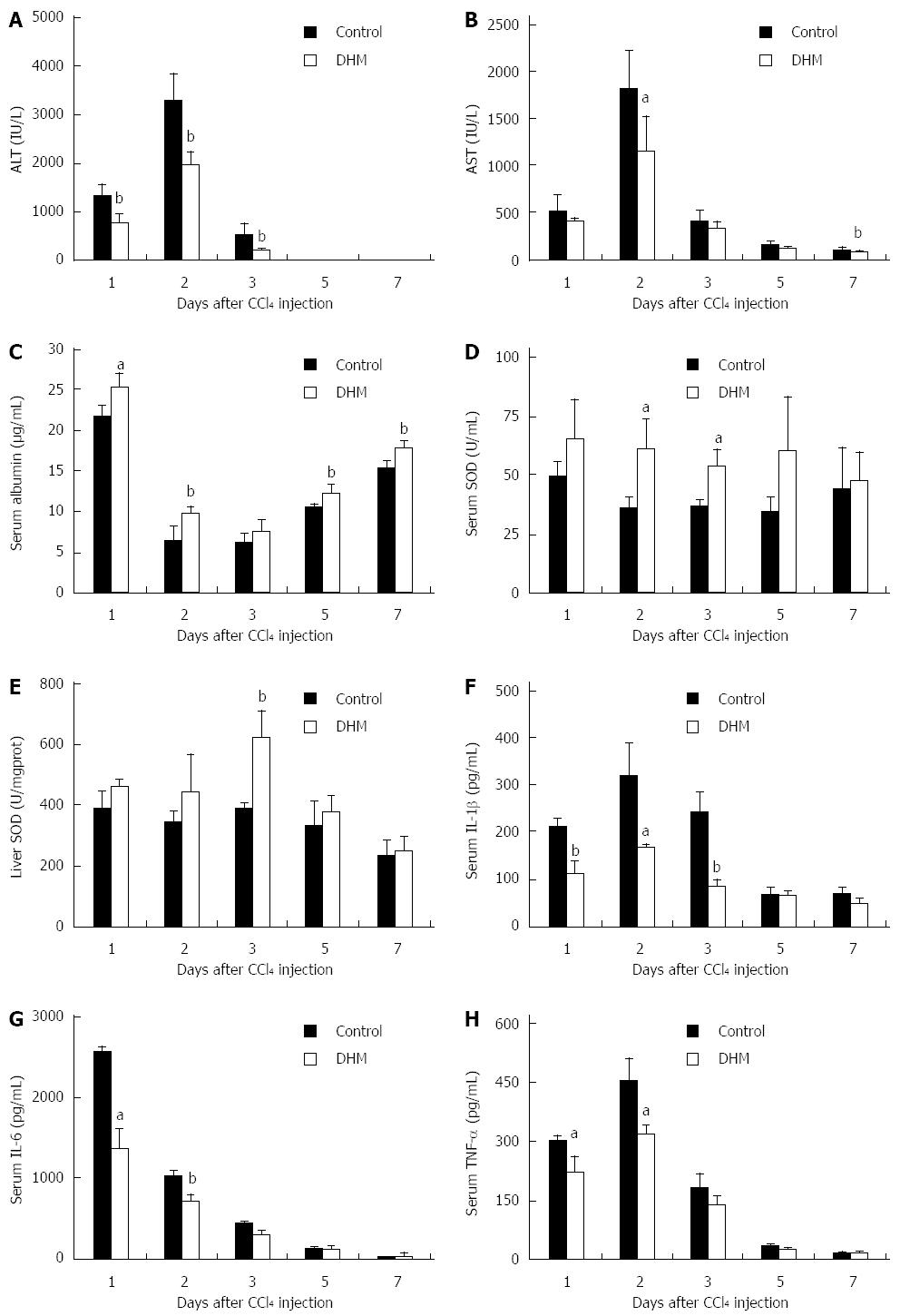
Figure 1 Dihydromyricetin protects mice against CCl4-induced liver injury.
Mice were treated with CCl4 (1 mL/kg body weight and 1:3 diluted in corn oil) to induce acute liver injury, and then orally administered with DHM (80 mg/kg body weight and diluted in CMC-Na) 2 h after CCl4 injection, once per day for 4 d. Control mice were treated with an equal volume of CMC-Na. Subsequently, serum ALT, AST, albumin, SOD, IL-1β, IL-6, TNF-α and liver SOD were measured at indicated time points and determined as described in materials and methods. A: Serum ALT; B: Serum AST; C: Serum Albumin; D: Serum SOD; E: Liver SOD; F: Serum IL-1β; G: Serum IL-6; H: Serum TNF-α. Values represent mean ± SE (n = 6). aP < 0.05 and bP < 0.01 vs control, respectively.
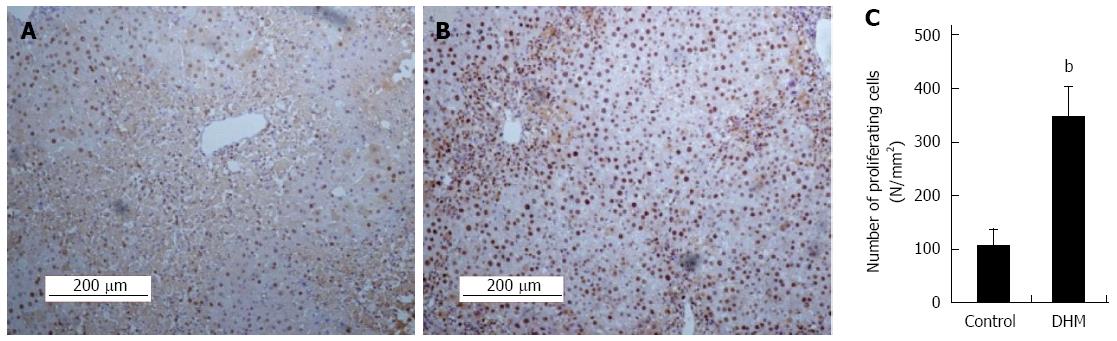
Figure 2 Dihydromyricetin accelerates liver regeneration in liver injury mice.
Immunohistochemical staining for proliferating cell nuclear antigen (PCNA) was carried out as previously described. Data showed PCNA staining liver sections in the mice without (A) or with dihydromyricetin (DHM) treatment (B) on day 2 after CCl4 injection. The control group showed few PCNA+ hepatocytes in centrilobular areas, but DHM administration resulted in numerous PCNA+ hepatocytes surrounding the edge of hepatocellular necrosis. (C) The numbers of PCNA+ cells. At least six 12 mm2 tissue sections were counted for each mouse. Values represent mean ± SE (n = 6), bP < 0.01 vs control.
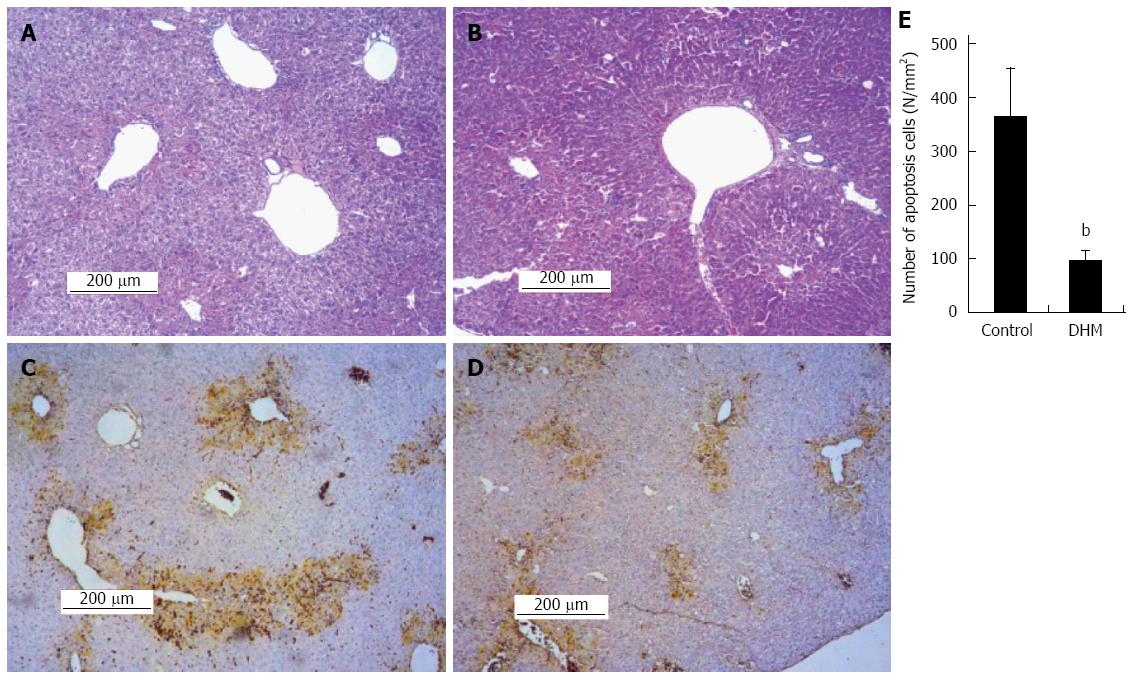
Figure 3 Dihydromyricetin reduces necrosis and inhibits apoptosis of hepatocytes in mice.
A: Representative liver section of the control group was stained with hematoxylin and eosin (HE) on day 2 after CCl4 injection, which shows partial necrosis with clusters of inflammatory cells around the central vein; B: Representative HE stained liver section of DHM administered mice on day 2 after CCl4 injection, which demonstrates decreased inflammatory cells and histological recovery with only inconspicuous necrosis still remaining around the central vein. In the TUNEL assay earthy yellow cells indicate apoptotic cells; C: The control group; D: Dihydromyricetin (DHM) treated group; E: The numbers of apoptotic cells. At least six 12 mm2 tissue sections were counted for each mouse. Values represent mean ± SE (n = 6), bP < 0.01 vs control.
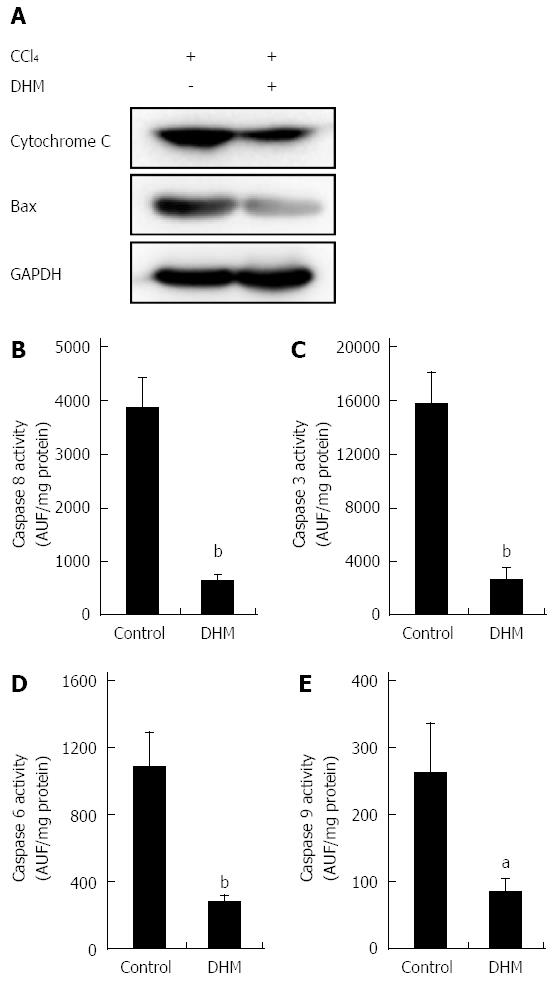
Figure 4 Dihydromyricetin reduces the expression of apoptosis-related proteins.
A: The release of cytochrome c from the mitochondria into cytosol and Bax expression in the DHM group were significantly inhibited compared with the control on day 2 after CCl4 (1 mL/kg) injection. Activities of Caspase 8 (B), Caspase 3 (C), Caspase 6 (D) and Caspase 9 (E) in the liver were markedly lower in mice of the DHM group than the control on day 2 after CCl4 (1 mL/kg) injection. Values represent mean ± SE (n = 6). aP < 0.05 and bP < 0.01 vs control, respectively.
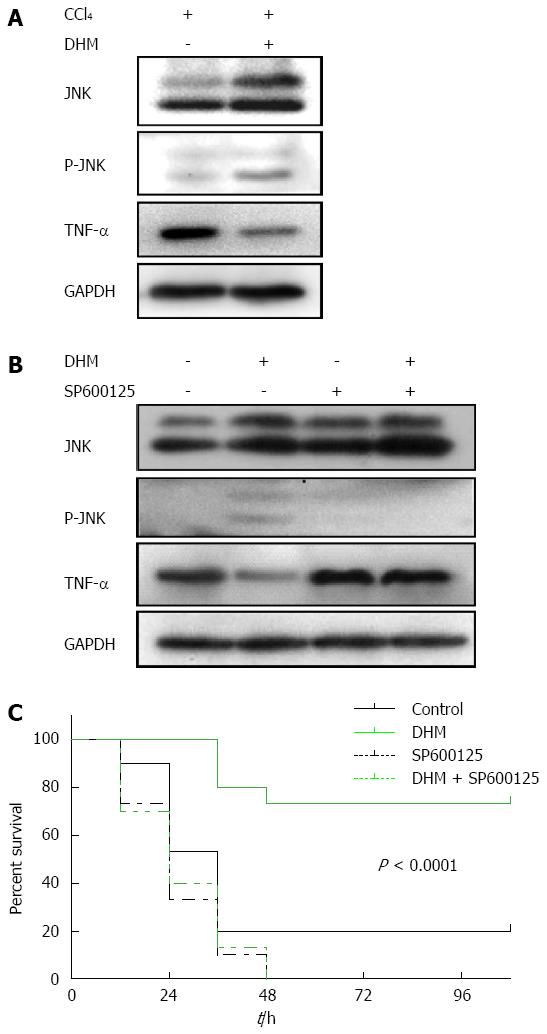
Figure 5 Dihydromyricetin protects from CCl4-induced acute liver failure by up-regulating JNK activation both in liver injury mice and acute liver failure mice.
A: Western blots depicting JNK pathway proteins on day 2 after CCl4 (1 mL/kg) injection; B: Western blots depicting JNK pathway proteins on day 2 after CCl4 (2.6 mL/kg) injection with or without of SP600125 treatment; C: The survival curves of the different conditions (n = 30/group).
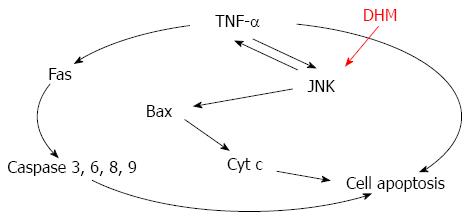
Figure 6 Mechanism of dihydromyricetin-triggered regulation of liver regeneration.
-
Citation: Xie J, Liu J, Chen TM, Lan Q, Zhang QY, Liu B, Dai D, Zhang WD, Hu LP, Zhu RZ. Dihydromyricetin alleviates carbon tetrachloride-induced acute liver injury
via JNK-dependent mechanism in mice. World J Gastroenterol 2015; 21(18): 5473-5481 - URL: https://www.wjgnet.com/1007-9327/full/v21/i18/5473.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i18.5473