Copyright
©2014 Baishideng Publishing Group Inc.
World J Gastroenterol. Aug 28, 2014; 20(32): 11400-11405
Published online Aug 28, 2014. doi: 10.3748/wjg.v20.i32.11400
Published online Aug 28, 2014. doi: 10.3748/wjg.v20.i32.11400
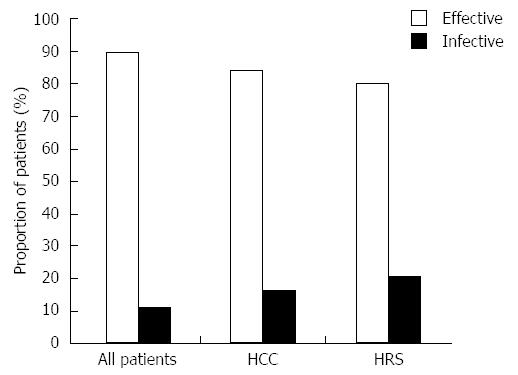
Figure 1 Overall efficacy of 15 mg/d tolvaptan.
Overall efficacy of 15 mg/d tolvaptan for 5-14 d in all patients as well as in subgroups of patients with coexisting hepatocellular carcinoma (HCC) and hepatorenal syndrome (HRS) post-tolvaptan treatment.
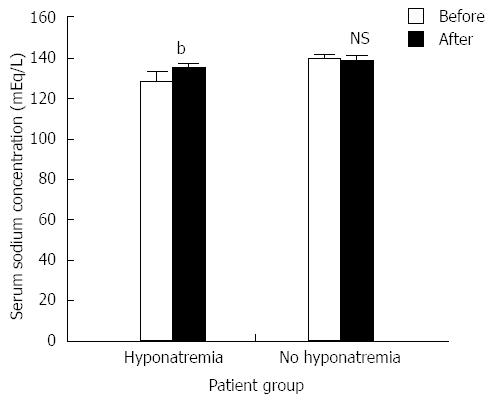
Figure 2 The effects of tolvaptan on serum sodium (mean ± SE) in patients with and without hyponatremia (n = 21 and n = 18, respectively).
Tolvaptan (15 mg/d, 5-14 d) treatment (black column) significantly increased serum sodium concentration [t (40) = -4.029, bP < 0.01 vs before treatment group] in patients with hyponatremia, but not in patients without hyponatremia [t (32) = 1.545, P > 0.05 (NS)]. NS: Not significant.
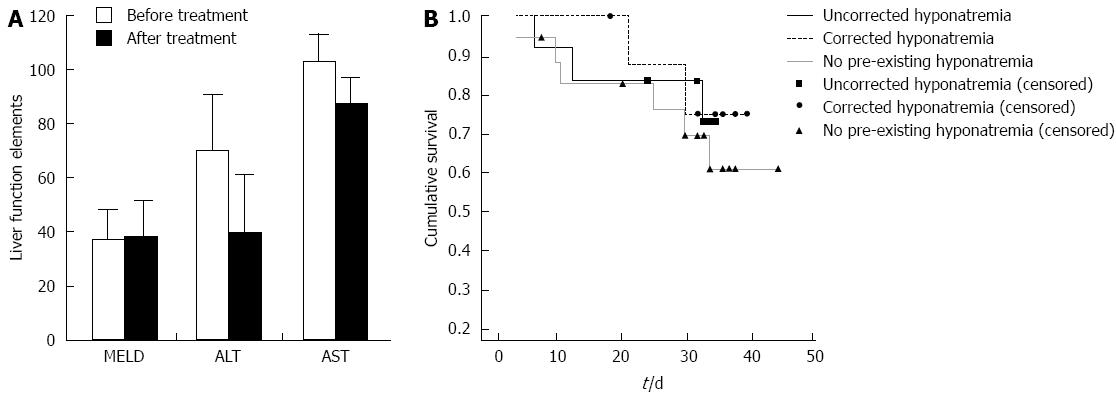
Figure 3 Tolvaptan does not affective liver function.
A: Tolvaptan (15 mg/d for 5-14 d) does not affective liver function [model for end-stage liver disease (MELD) score, alanine aminotransferase (ALT) and aspartate aminotransferase (AST)] in patients with preexisting liver cirrhosis (P > 0.05); B: Kaplan-Meier analysis of the 1-mo survival of patients without hyponatremia, as well as with and without short-term correction of hyponatremia, did not show any significant difference between patient groups.
- Citation: Zhang X, Wang SZ, Zheng JF, Zhao WM, Li P, Fan CL, Li B, Dong PL, Li L, Ding HG. Clinical efficacy of tolvaptan for treatment of refractory ascites in liver cirrhosis patients. World J Gastroenterol 2014; 20(32): 11400-11405
- URL: https://www.wjgnet.com/1007-9327/full/v20/i32/11400.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i32.11400