Copyright
©2014 Baishideng Publishing Group Inc.
World J Gastroenterol. Jun 28, 2014; 20(24): 7707-7717
Published online Jun 28, 2014. doi: 10.3748/wjg.v20.i24.7707
Published online Jun 28, 2014. doi: 10.3748/wjg.v20.i24.7707
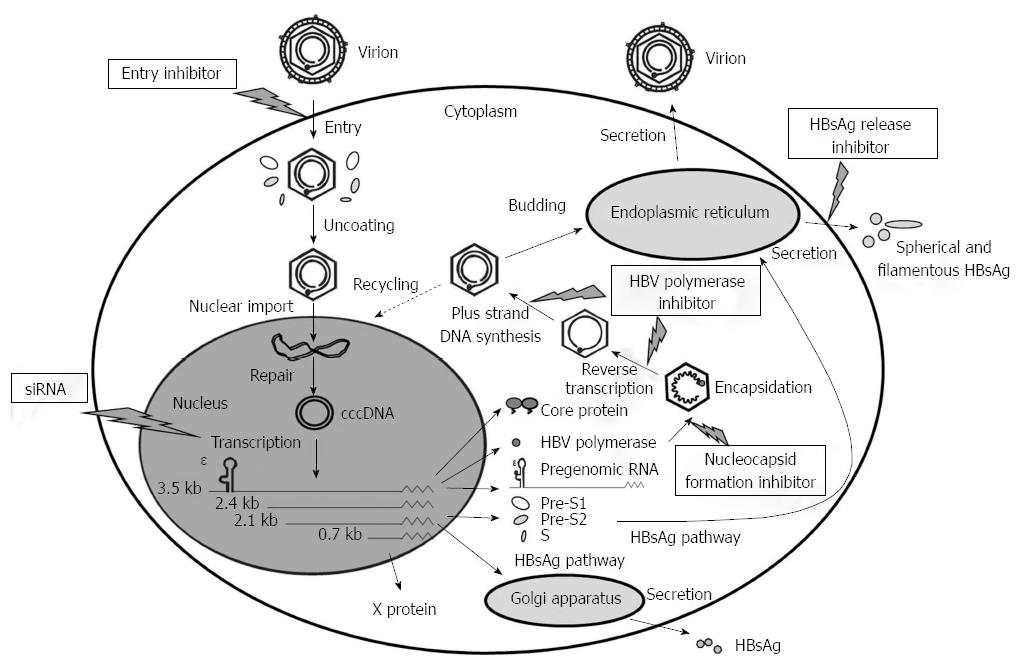
Figure 1 Hepatitis B virus replication cycle and potential drug targets.
After hepatitis B virus (HBV) virions enter the hepatocyte, they are uncoated and transported to the nucleus, in which covalently closed circular DNA (cccDNA) is generated by repairing the partially double-stranded genome, followed by transcription of the cccDNA into the viral RNA gene products. Encapsidation of the pregenomic RNA occurs in the cytoplasm via a complex interaction among epsilon, core particles, and HBV polymerase. Reverse transcription leading to negative-strand and then positive-strand synthesis occurs within the viral nucleocapsid. After budding into the endoplasmic reticulum and Golgi apparatus, the nucleocapsid acquires an HBV surface antigen (HBsAg)-containing envelope and is released through the secretory pathway as progeny virions. Alternatively, nucleocapsids may also recycle their genomes into the nucleus for conversion to cccDNA. Potential inhibitors of the HBV life cycle include inhibitor of viral entry, small interfering double-stranded RNA (siRNA), inhibitor of nucleocapsid formation, inhibitor of HBV DNA polymerase, and inhibitor of HBsAg release.
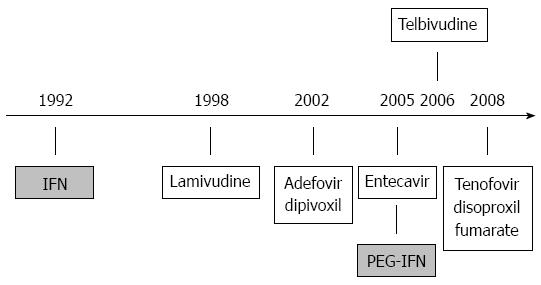
Figure 2 Approval dates for chronic hepatitis B therapeutics.
IFN: Interferon.
- Citation: Wang XY, Chen HS. Emerging antivirals for the treatment of hepatitis B. World J Gastroenterol 2014; 20(24): 7707-7717
- URL: https://www.wjgnet.com/1007-9327/full/v20/i24/7707.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i24.7707