Copyright
©2014 Baishideng Publishing Group Co.
World J Gastroenterol. Apr 28, 2014; 20(16): 4681-4691
Published online Apr 28, 2014. doi: 10.3748/wjg.v20.i16.4681
Published online Apr 28, 2014. doi: 10.3748/wjg.v20.i16.4681
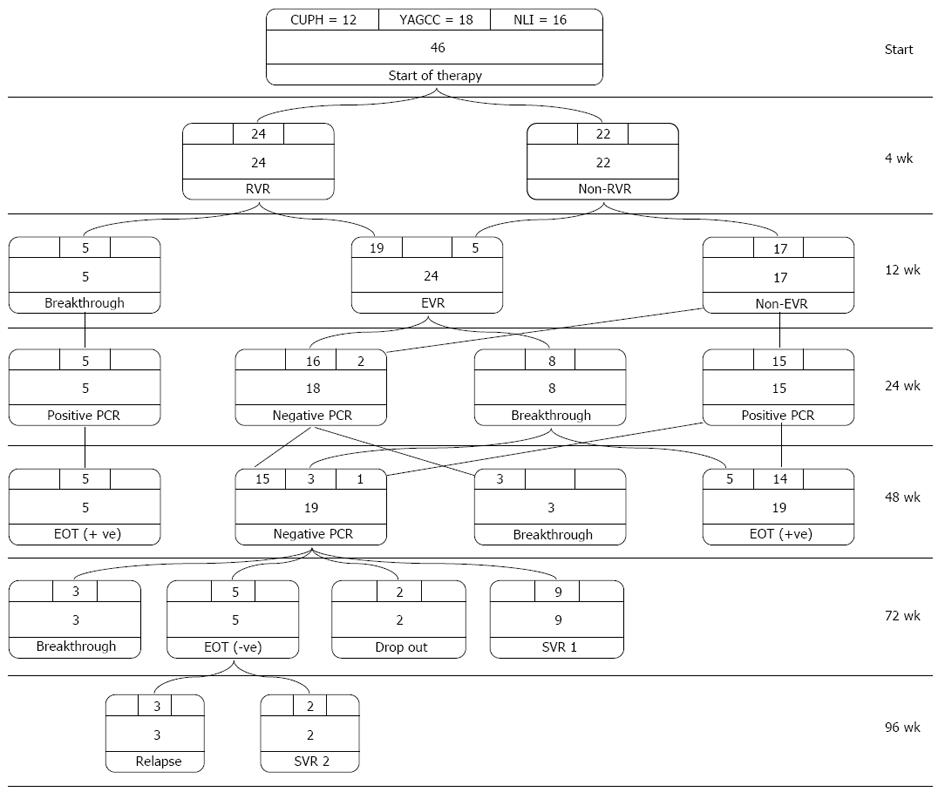
Figure 1 Treatment algorithm and response to therapy.
At weeks 4, 24 (52.17%) patients attained a rapid virological response (RVR) and 22 (47.82%) had no RVR. At week 12, five patients (10.86%) had breakthrough, while the remaining 19 patients had an early virological response (EVR). Those patients, in addition to five patients who turned negative from ones with no RVR formed a total of 24 (52.17%) patients with EVR. At week 24, those who had breakthrough continued to be polymerase chain reaction (PCR)-positive and eight patients from those with EVR had breakthrough. The remaining 16 patients, in addition to two patients from those with no EVR, turned PCR-negative, making a total of 18 (39.13%) patients with negative PCR. At week 48, three of those 18 patients had breakthrough, while the remaining 15 patients and one patient from those positive at week 24, in addition to three patients who had breakthrough at week 24 (making a total of 19 patients), had negative PCR. Of the 19 negative-PCR patients, nine had an SVR at week 72 and two patients dropped out. The remaining eight patients had an extended 6-month therapy (three patients had breakthrough and five patients were PCR-negative at their ETR). At week 96, three out of five patients with extended course had relapse, while the other two patients attained an SVR, making the total SVR 11 out of 44 (25%). EOT: End of treatment.
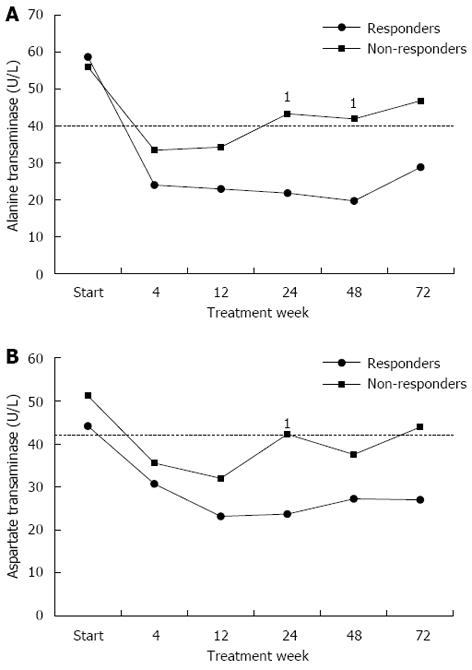
Figure 2 Alanine aminotransferase and aspartate aminotransferase mean levels during treatment in responders and non-responders.
A: Alanine aminotransferase (ALT) mean levels remained normal in responders all through the follow up period, while in non-responders, after starting as normal, they increased again to significantly higher levels than in responders from week 12 onwards, especially at weeks 24 and 48 (1P = 0.007, 0.003 respectively); B: Aspartate aminotransferase (AST) mean levels remained normal in responders all through the follow up period, while in non-responders, after starting as normal, they significantly increased again in week 24 (1P = 0.007) and once more in week 72. The dashed line represents the upper limit of normal.
-
Citation: Naghi SE, Abdel-Ghaffar TY, El-Karaksy H, Abdel-Aty EF, El-Raziky MS, Allam AA, Helmy H, El-Araby HA, Behairy BE, El-Guindi MA, El-Sebaie H, Abdel-Ghaffar AY, Ehsan NA, El-Hennawy AM, Sira MM. Safety and efficacy of
Hansenula -derived PEGylated-interferon alpha-2a and ribavirin combination in chronic hepatitis C Egyptian children. World J Gastroenterol 2014; 20(16): 4681-4691 - URL: https://www.wjgnet.com/1007-9327/full/v20/i16/4681.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i16.4681