Copyright
©2012 Baishideng Publishing Group Co.
World J Gastroenterol. Feb 14, 2012; 18(6): 522-531
Published online Feb 14, 2012. doi: 10.3748/wjg.v18.i6.522
Published online Feb 14, 2012. doi: 10.3748/wjg.v18.i6.522
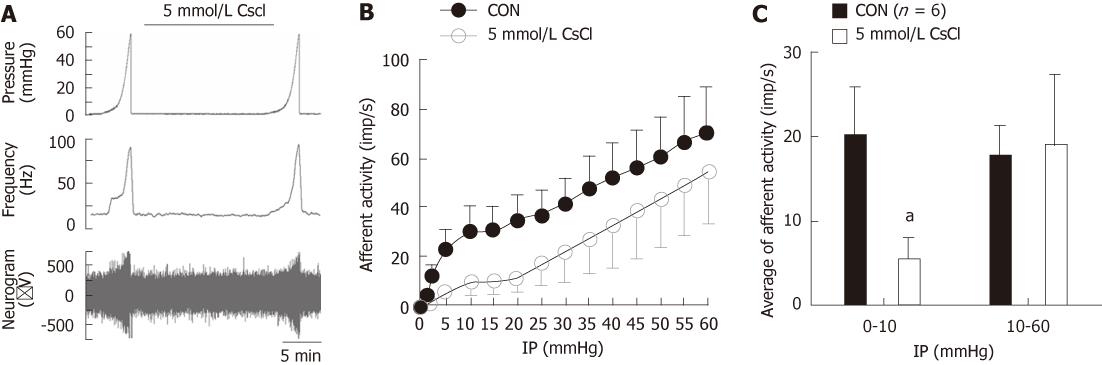
Figure 1 Effects of CsCl on the mechenosensory activity of small intestinal afferents.
A: Original recording of the mesenteric afferent nerve activity in response to distension in an ex vivo jejunum preparation; B: The pressure-afferent nerve response curves in control and in the presence of hyperpolarization-activated cyclic nucleotide-gated cation blocker, 5 mmol/L CsCl; C: Bar graph showing the low- and high- threshold mechanosensory responses with or without the presence of 5 mmol/L CsCl. aP < 0.05.
Figure 2 Effects of ZD-7288 on the mechanisensory responses of mesenteric afferent nerves.
Note that the slopes of the average pressure-response curves in the pressure range of 10 mmHg - 60 mmHg were apparently similar with or without the hyperpolarization-activated cyclic nucleotide-gated cation channel blocker ZD-7288 (10 μmol/L). Data were pooled from 3 preparations.
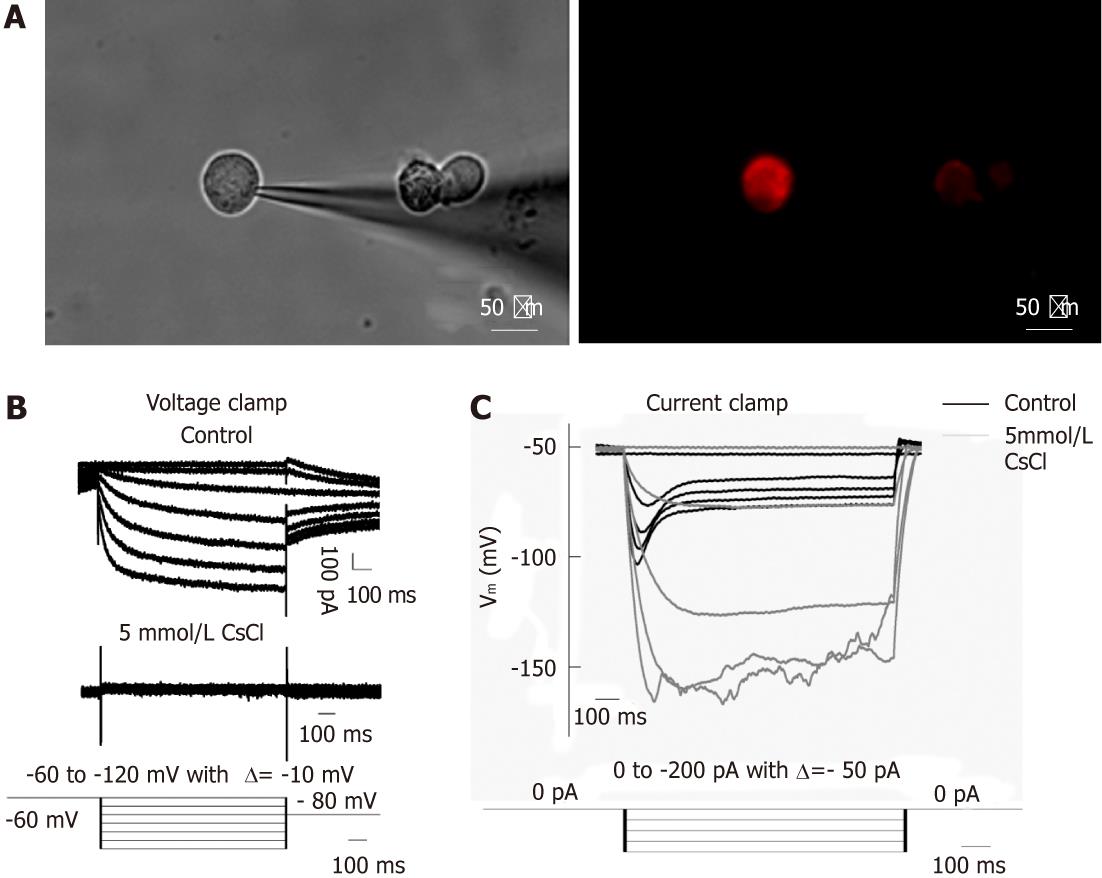
Figure 3 Ih recorded under whole-cell voltage and current clamp from DiI labeled dissociated dorsal root ganglia and nodose ganglia neurons innervating the jejunum.
A: DiI labeled dissociated dorsal root ganglia neurons; B: Representative current traces of Ih current (top trace); Current recorded in the presence of 5 mmol/L CsCl (middle trace); voltage clamp protocal: Ih was induced from a holding potential of -60 mV in 1 s pulses from -60 mV to -120 mV in steps of 10 mV, followed by a final step to -80 mV to record the tail current (bottom trace); C: Voltage response to test current pulse before (black) and after (grey) application of 5 mmol/L CsCl (top trace); bottom trace shows the current clamp protocol, i.e., hyperpolarizing current pulses ranging from 0 pA to -200 pA in steps of 50 pA. Note that the current elicited an instantaneous hyperpolarization that was followed by depolarization (named “sag”) of membrane potential. DRG: Dorsal root ganglia; NG: Nodose ganglia.
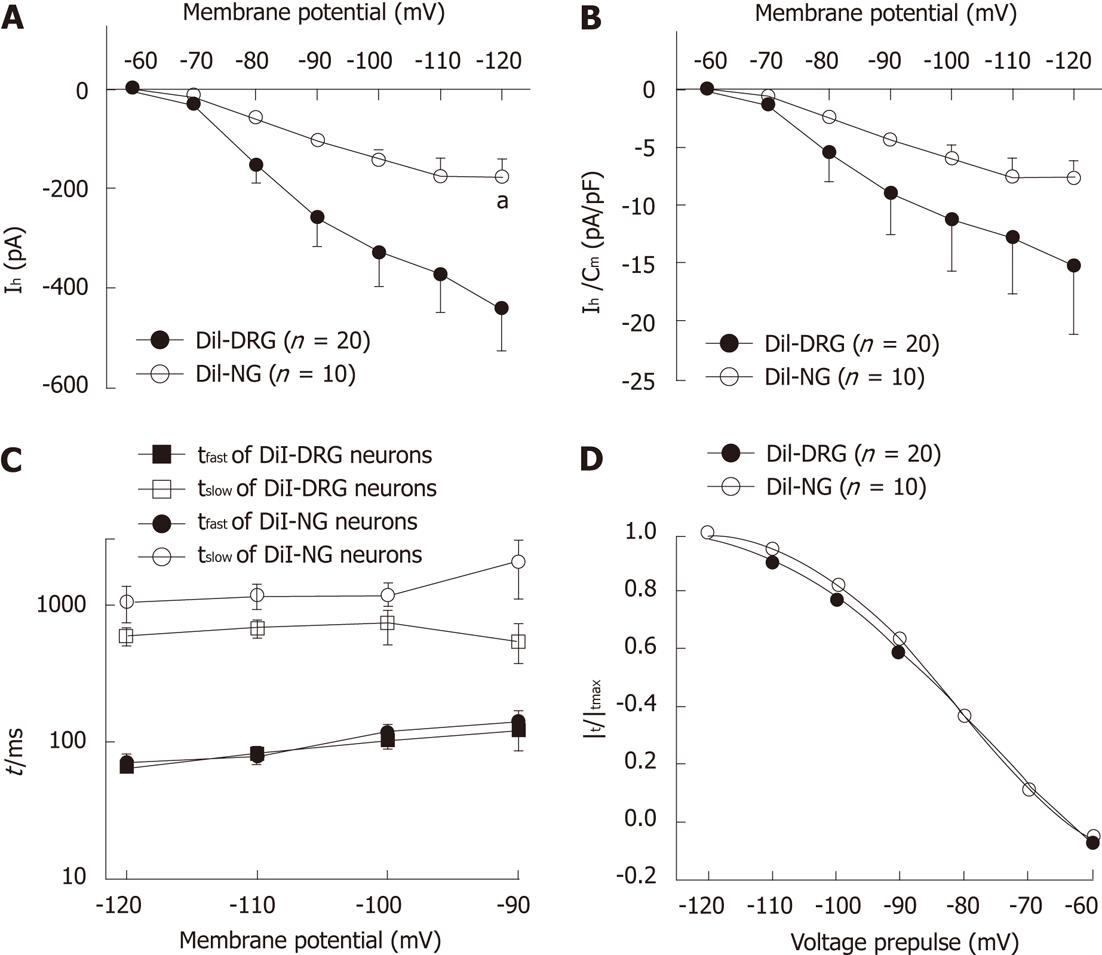
Figure 4 Ih steady-state parameters and activation kinetics in DiI-labeled DRG and NG neurons.
A: Ih current-voltage relationship between DiI-labeled dissociated dorsal root ganglia (DRG) and nodose ganglia neurons (NG); B: Ih current density-voltage relationship between DiI-labeled DRG and NG neurons; C: The time constant of Ih current. Ih current traces were fitted with two exponentials according to the following equation: Ih(t) = Af exp(-t/τf) + As exp(-t/τs), where Ih(t) is the amplitude of the current at time t and Af and As are the initial amplitudes of the fast (τf) and slow (τs) activation time constant components, respectively. Time constants were obtained by fitting currents using pCLAMP; D: Ih current activation curves. Normalized activation curves were obtained from tail currents at -80 mV and fitted by Boltzmann function: Ih/Ih(max) =1/{1+ exp[(Vm – V1/2)/k]}, where Ih is the peak amplitude of the tail current recorded immediately after the pre-pulse, Ih(max) is the maximal current recorded after the maximal prepulse of -120 mV, Vm is the membrane potential, V1/2 is the membrane potential at which Ih conductance is half-activated, and k is a slope factor of the curve. Data were expressed as mean ± SE. aP < 0.05 vs DiI-labeled DRG neurons.
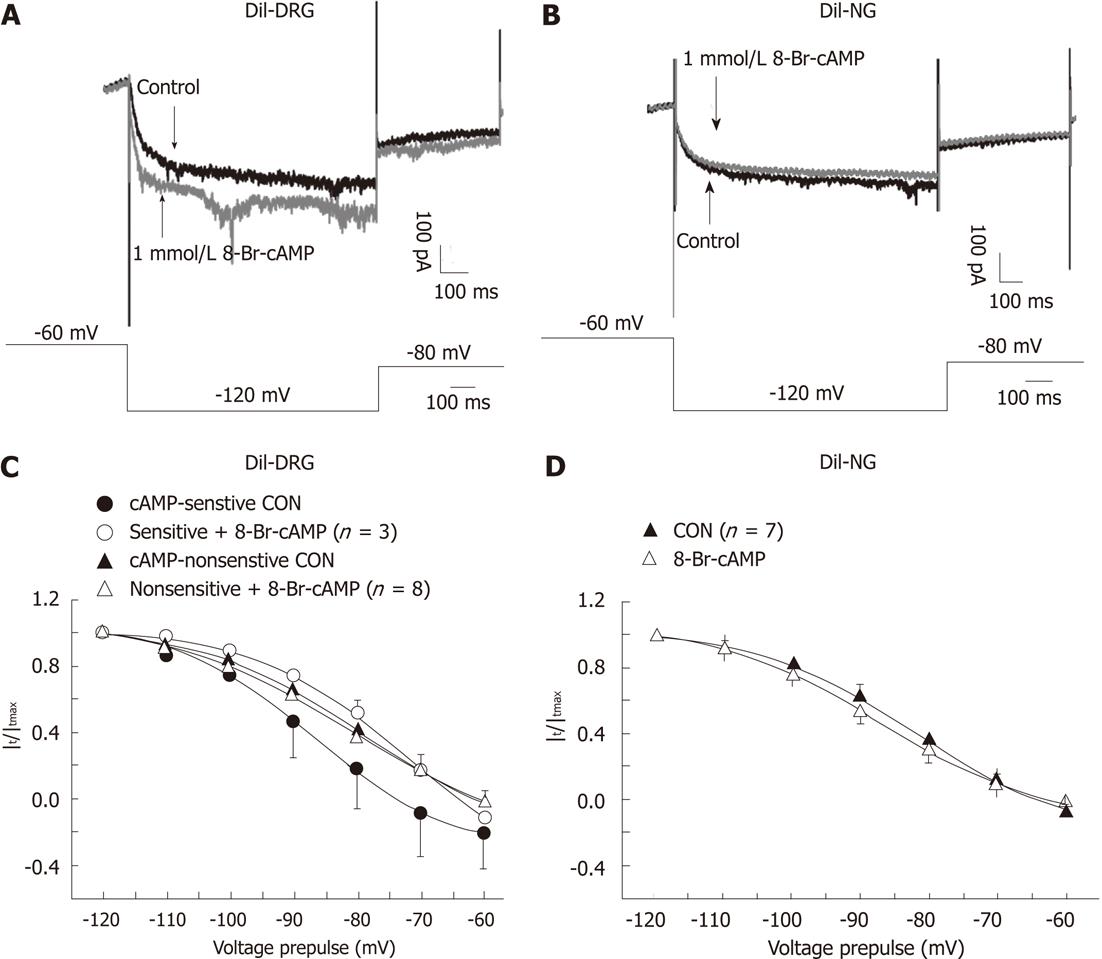
Figure 5 Effects of 8-Br-cAMP on the activation curves of Ih current in DiI-labeled dissociated dorsal root ganglia and nodose ganglia neurons.
A and B: Overdraw of the Ih current traces elicited by a hyperpolarizing pulse (-120 mV) in control and in the presence of 8-Br-cAMP in DiI-labeled dissociated dorsal root ganglia (DRG) (A) and nodose neurons (B); C: Activation curves of cAMP-sensitive and cAMP-insensitive DiI-labeled DRG neurons before (black block symbols) and after treatment with 8-Br-cAMP (empty symbols); D: Activation curves of DiI-labeled nodose ganglia neurons before (black block triangle) and after treatment with 8-Br-cAMP (empty triangle). Data were expressed as mean ± SE.
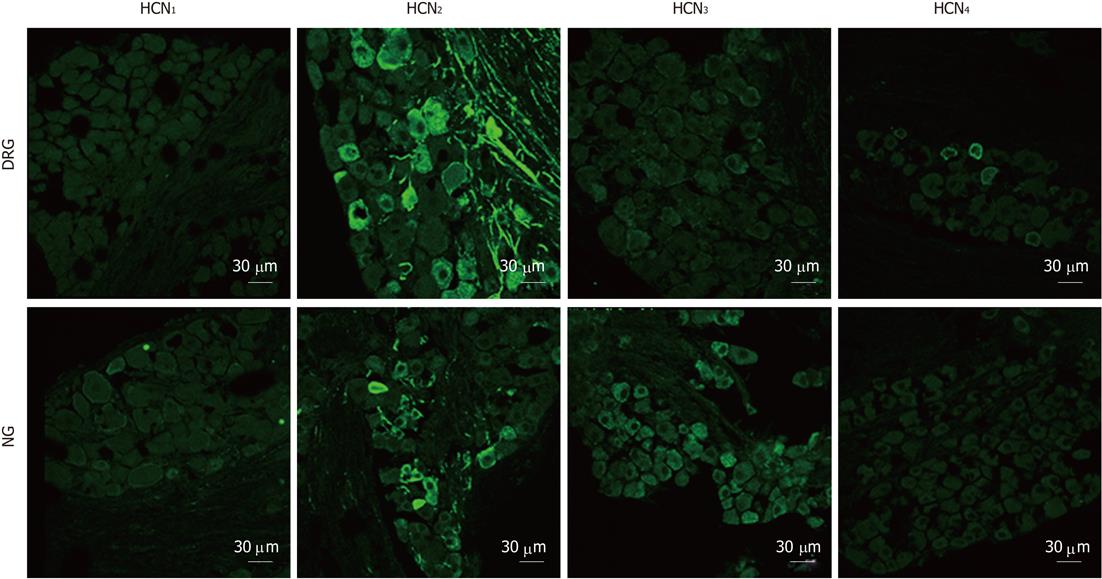
Figure 6 Immunostaining for hyperpolarization-activated cyclic nucleotide-gated cation isoforms in dorsal root ganglia and nodose ganglia sections.
Hyperpolarization-activated cyclic nucleotide-gated cation channel 1 (HCN1) immunoreactivity was not detectable in dorsal root ganglia (DRG) and nodose ganglia (NG) neurons. HCN2 immunoreactivity was prominent in DRG (cell bodies and fibers) and was also present in some NG neurons and fibers. HCN3 immunoreactivity was profuse in NG sections but was weaker in DRG sections. HCN4 immunoreactivity was present in a minority of DRG neurons and was absent in NG.
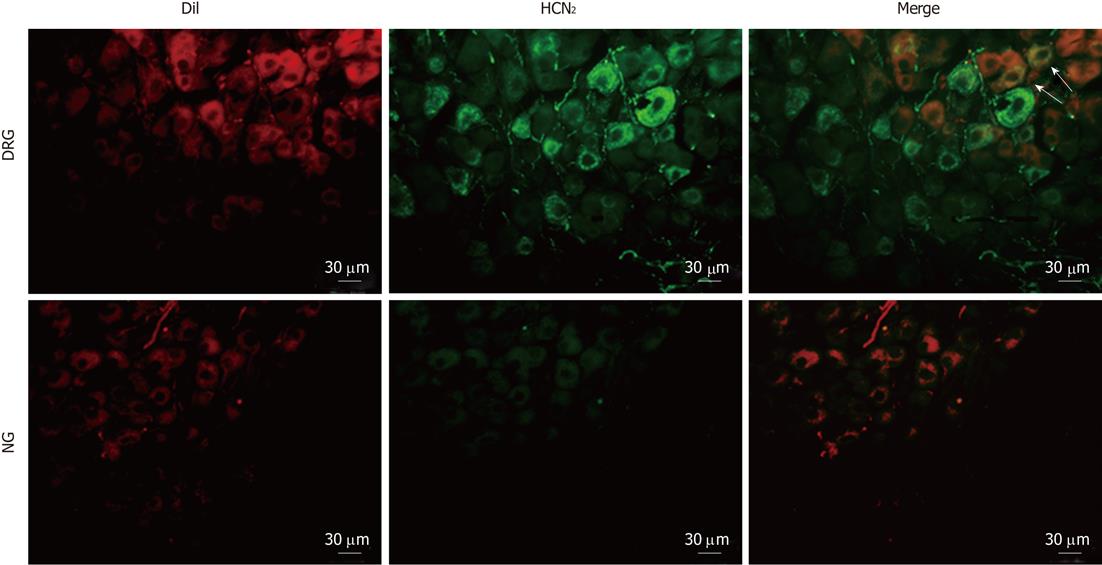
Figure 7 Immunostaining for hyperpolarization-activated cyclic nucleotide-gated cation channel 2 in intestinal primary afferent neurons.
Spinal and vagal afferent neurons innervating the small intestine were labeled via injection of DiI (red) into the gut wall. Arrows indicate positive hyperpolarization-activated cyclic nucleotide-gated cation channel 2 (HCN2) staining in DiI-labeled dorsal root ganglia neurons. DRG: Dissociated dorsal root ganglia; NG: Nodose ganglia.
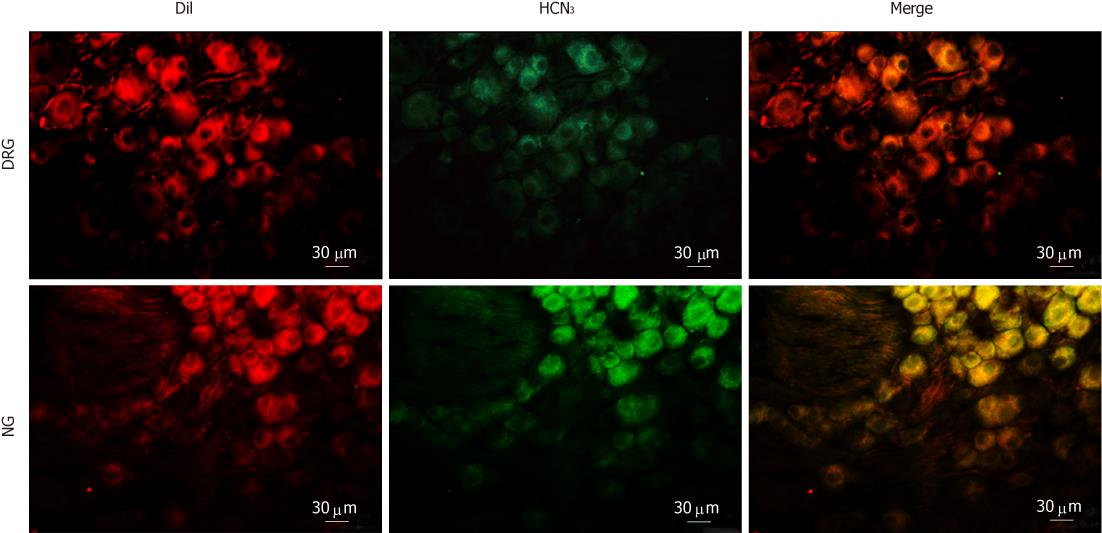
Figure 8 Immunostaining for hyperpolarization-activated cyclic nucleotide-gated cation channel 3 in intestinal primary afferent neurons.
Intestinal afferents were labeled via injecting DiI into the gut wall. Note that weak hyperpolarization-activated cyclic nucleotide-gated cation channel 3 (HCN3) immunoreactivity (green) was present in some DiI-labeled (red) dorsal root ganglia neurons. In contrast, most DiI-labeled nodose ganglia neurons were moderately stained for HCN3.
- Citation: Wang YP, Sun BY, Li Q, Dong L, Zhang GH, Grundy D, Rong WF. Hyperpolarization-activated cyclic nucleotide-gated cation channel subtypes differentially modulate the excitability of murine small intestinal afferents. World J Gastroenterol 2012; 18(6): 522-531
- URL: https://www.wjgnet.com/1007-9327/full/v18/i6/522.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i6.522