Copyright
©2011 Baishideng Publishing Group Co.
World J Gastroenterol. Jul 28, 2011; 17(28): 3300-3309
Published online Jul 28, 2011. doi: 10.3748/wjg.v17.i28.3300
Published online Jul 28, 2011. doi: 10.3748/wjg.v17.i28.3300
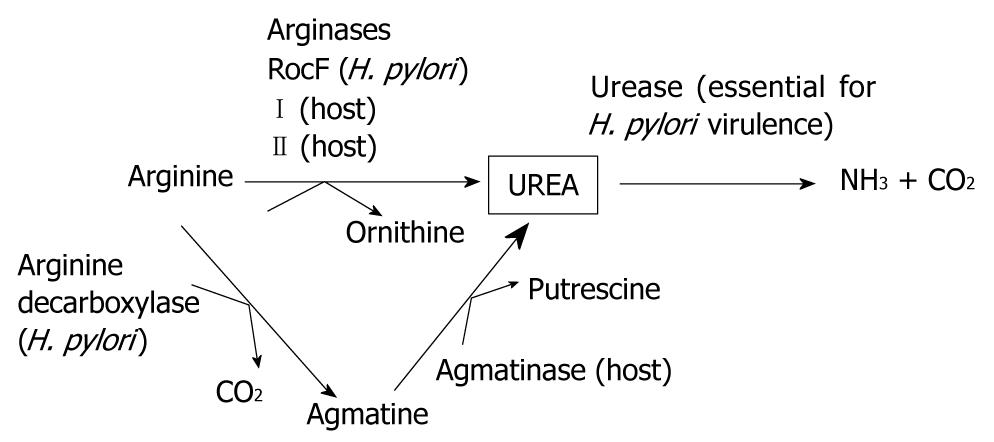
Figure 1 Overview of possible urea sources for Helicobacter pylori urease.
Helicobacter pylori (H. pylori) urease is essential for colonization of mice, which indicates that the substrate for the enzyme, urea, is also essential. This study examines the three possible arginase-mediated sources for the urea (underlined): arginase from H. pylori (RocF), arginase I (host) and arginase II (host). Another possible urea source is host agmatinase. H. pylori does not have an agmatinase, but does have an enzyme, arginine decarboxylase, that can synthesize agmatine. Arginine is an essential amino acid for H. pylori and serves as a substrate for both arginase and arginine decarboxylase.
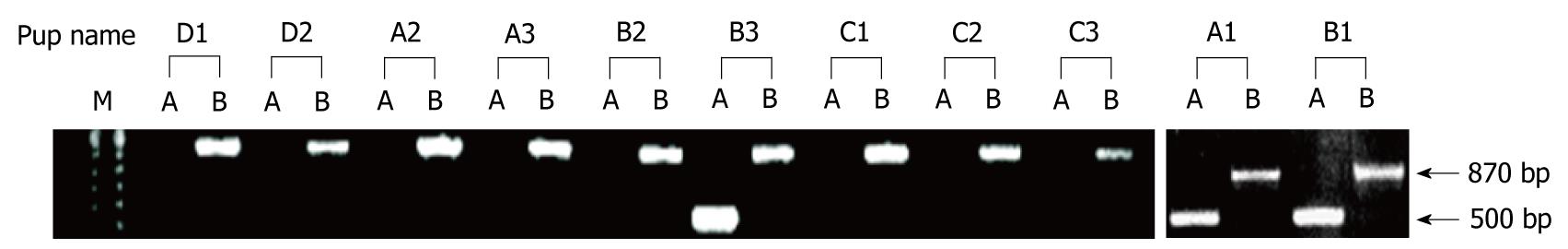
Figure 2 Genotyping of mice to determine whether they are heterozygous or homozygous arginase II knockouts.
Pups obtained from breeding heterozygous males to arginase II knockout females were genotyped by PCR analysis. Wild-type band = 500 bp (“A” lanes); mutant band = 870 bp (“B” lanes). Eight of the 11 pups were homozygous knockouts. Pups A1, B1, and B3 were heterozygous because they contained wild-type and mutant copies of arginase II. M: 100 bp marker (Bio-Rad).
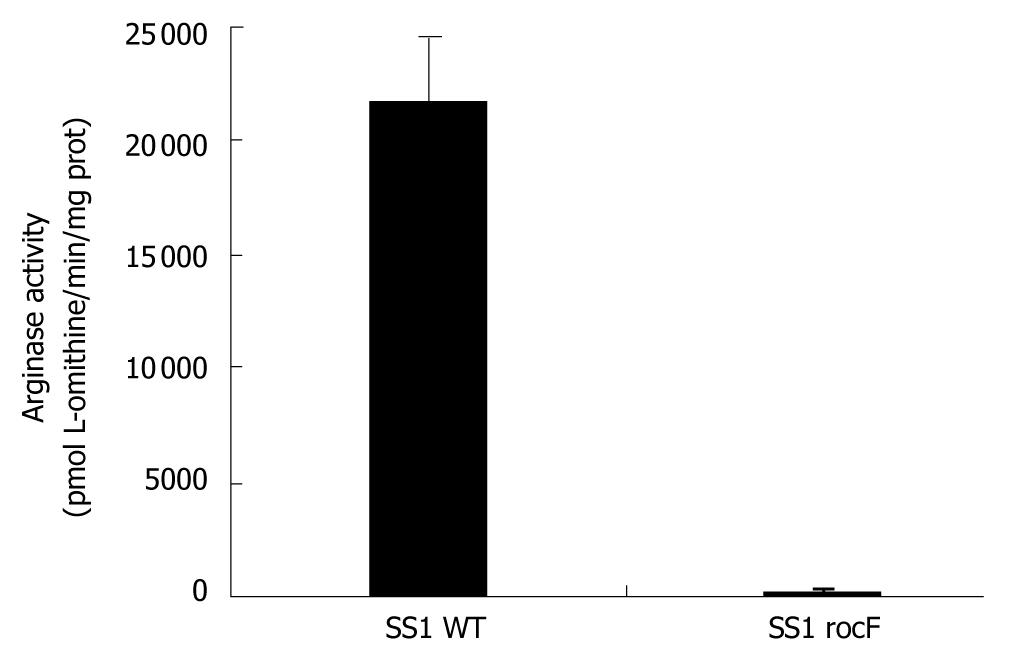
Figure 3 The rocF mutant of Helicobacter pylori strain SS1 is devoid of arginase activity.
Bacteria were harvested in 0.9% NaCl and ice-bath-sonicated (25% intensity, 2 pulses of 30 s each, with 30 s rests on ice between pulses). Following centrifugation (12 000 g, 2 min, 4°C), supernatants were retained on ice. Equal volumes of extract and 10 mmol/L cobaltous chloride (CoCl2•6 H2O, final concentration of 5 mmol/L) were preincubated for 30 min at 50-55°C to activate the enzyme. Arginase buffer (15 mmol/L Tris, pH 7.5, or 15 mmol/L MES, pH 6.0, plus 10 mmol/L L-arginine) was added and incubated at 37°C for 1 h. The reaction was stopped by addition of 750 μL acetic acid, and the color developed by addition of 250 μL ninhydrin (4 mg/mL) at 95°C for 1 h and read at 515 nm. The data are presented in units where 1 U is defined as one pmol L-ornithine/min/mg protein + SD. Details of this assay have been reported previously[23].
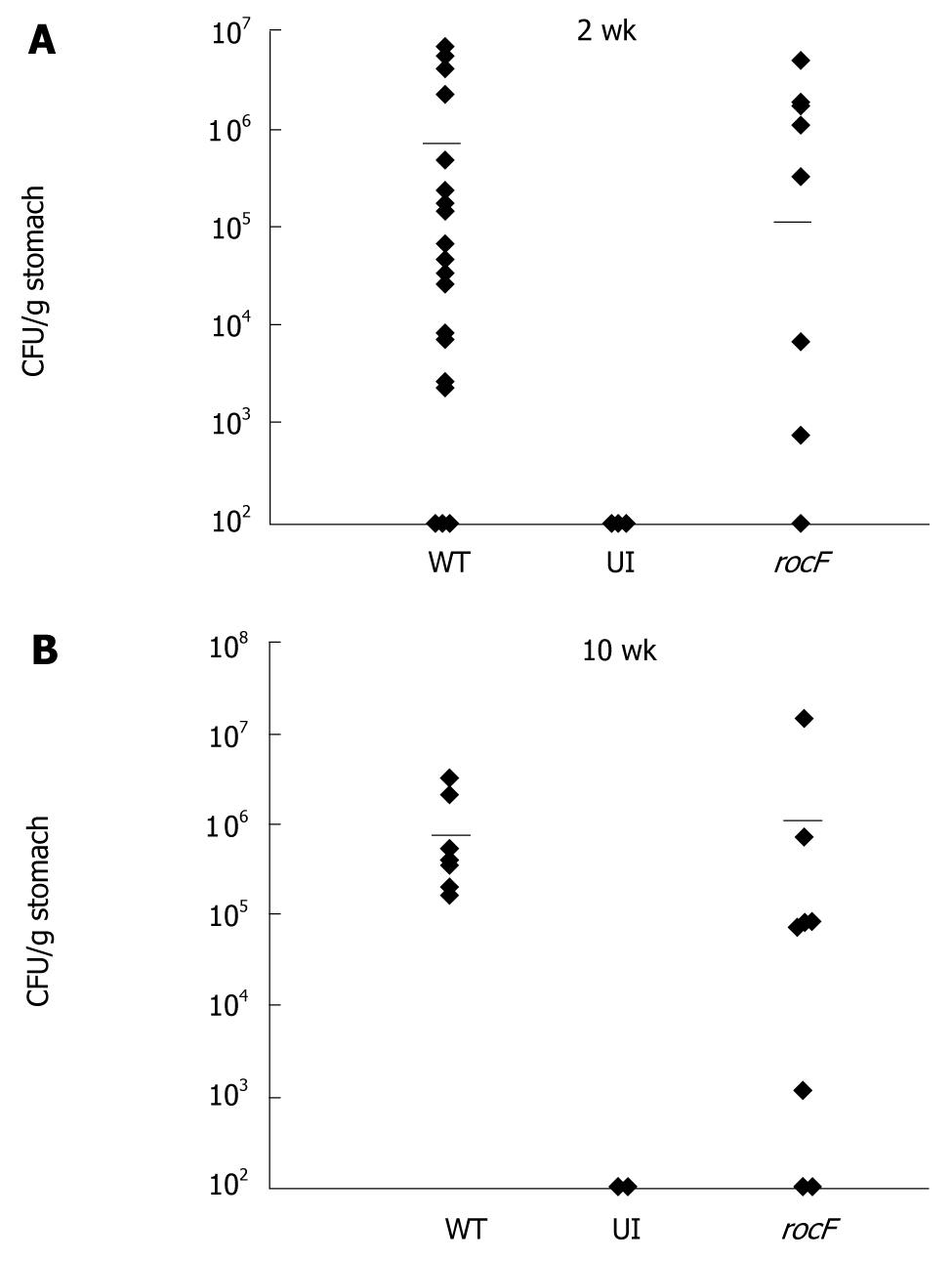
Figure 4 The rocF mutant of Helicobacter pylori colonizes the stomach of arginase II knockout mice.
Arginase II knockout mice were inoculated with either the wild-type SS1 strain (WT) of Helicobacter pylori (H. pylori) or the isogenic rocF mutant (rocF). Number of animals used per group were as follows: 2 wk experiment: 13 for WT, nine for rocF mutant; 10 wk experiment: eight for WT, eight for rocF mutant. At 2 (A) or 10 (B) wk post-infection, stomachs were removed, completely homogenized, and plated for H. pylori. UI, uninfected controls (n = 2 at 2 and 10 wk). Limit of detection: ~100 CFU/g stomach; all animals that lacked H. pylori were set to this detection limit. Bar, mean CFU/g stomach. At 2 wk post-infection, P = 0.65 by unpaired two-tailed t test between the mean CFU/g tissue for the wild-type versus the rocF mutant. Each symbol represents one mouse. In some cases there are two mice represented by a symbol if the data overlapped.
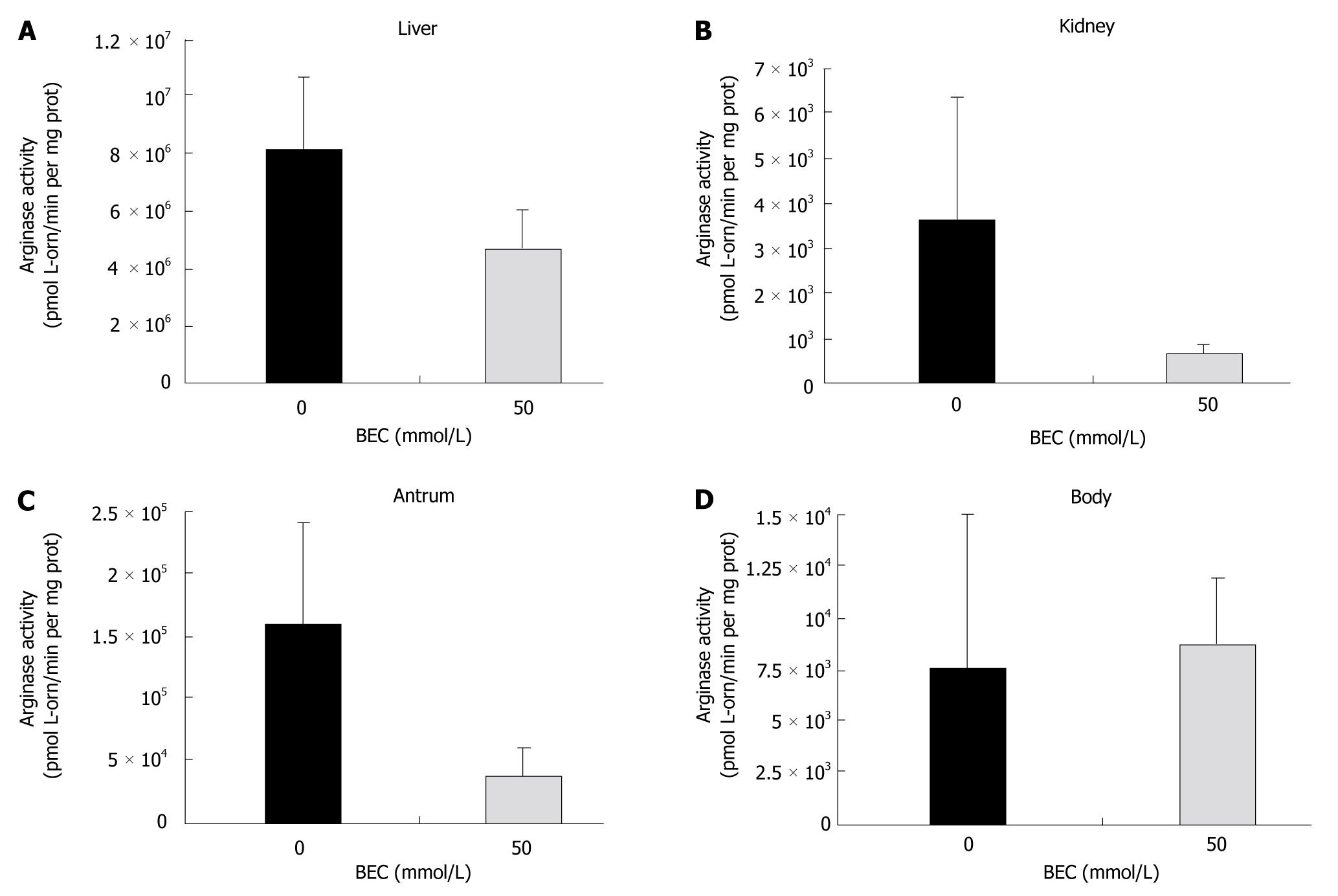
Figure 5 Ex vivo arginase activity of organs from arginase II knockout mice fed water or the arginase inhibitor S-(2-boronoethyl)-L-cysteine.
Mice were administered 20 μL water or 50 mmol/L S-(2-boronoethyl)-L-cysteine (BEC) once or twice daily for 3 d by direct oral delivery via pipetting. Animals were euthanized and organs removed and homogenized. The data represent the average arginase activity of four to five mice ± SD, with each mouse organ measured in duplicate or triplicate using arginine buffer at pH 9.0 in the presence of manganese. A: Liver. Arginase activity was inhibited almost 50% in the liver by BEC. P = 0.0006 between the two groups; B: Kidney. Arginase activity was inhibited > 75% in the kidney by BEC. P = 0.0033 between the two groups; C: Antrum. Arginase activity was inhibited > 75% in the antrum by BEC. P = 0.0007 between the two groups; D: Body. No inhibition was observed in the body (P > 0.05).
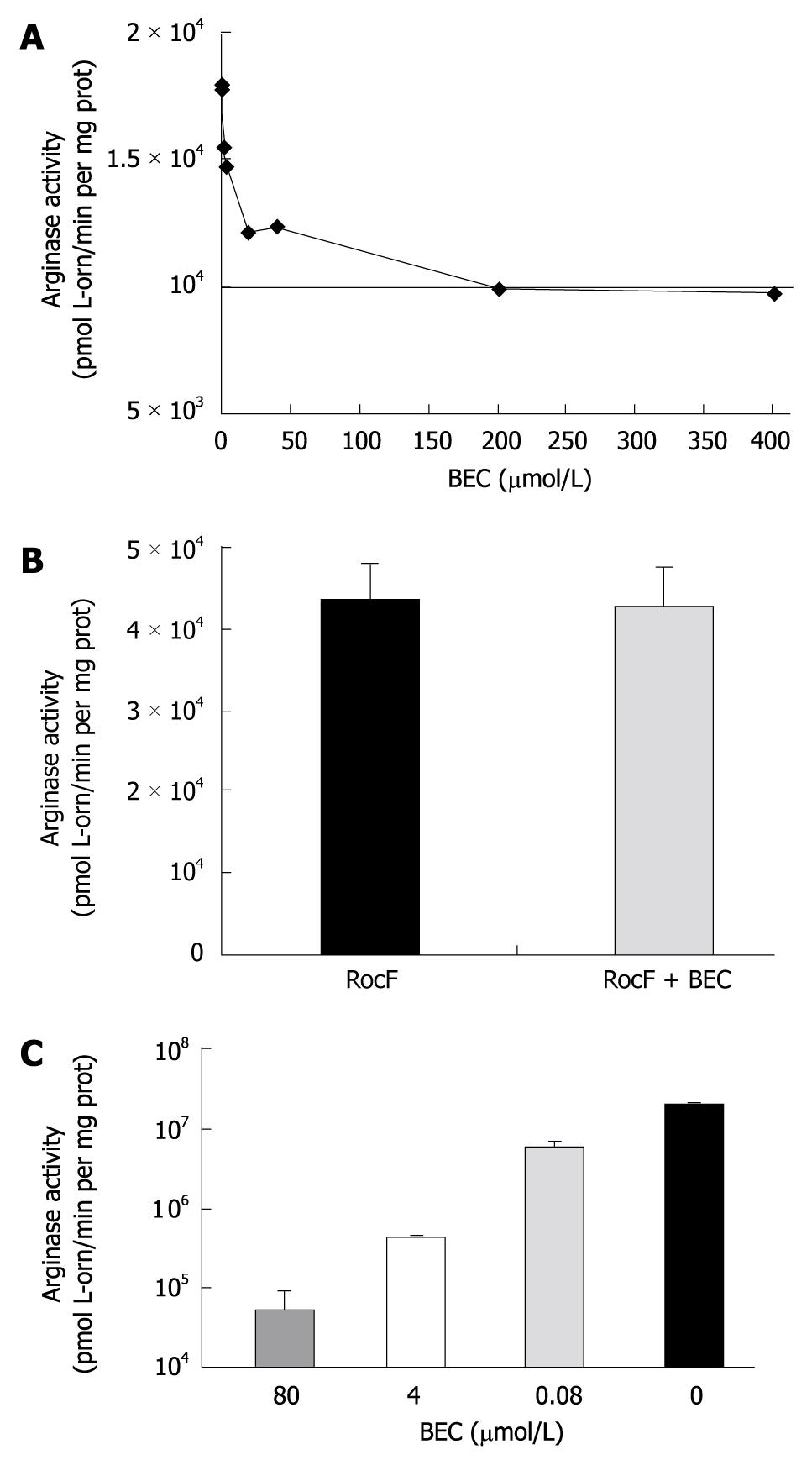
Figure 6 S-(2-boronoethyl)-L-cysteine does not strongly inhibit Helicobacter pylori arginase activity in vitro.
A: Arginase-containing extracts from Escherichia coli (E. coli) expressing the Helicobacter pylori rocF gene on pGEN222. Extracts were incubated in the presence or absence of various concentrations of S-(2-boronoethyl)-L-cysteine (BEC) and measured for arginase activity at pH 6.0 in the presence of cobalt; B: Purified arginase (His6-RocF) was incubated in the presence or absence of BEC (400 nmol/L) and assayed for arginase activity in the presence of cobalt at pH 6.0; C: Extracts from E. coli expressing rat arginase I were prepared and incubated in the presence of different concentrations of BEC and then assayed for arginase activity in the presence of manganese at pH 9.0. Graph plotted on a logarithmic scale due to magnitude of arginase activity.
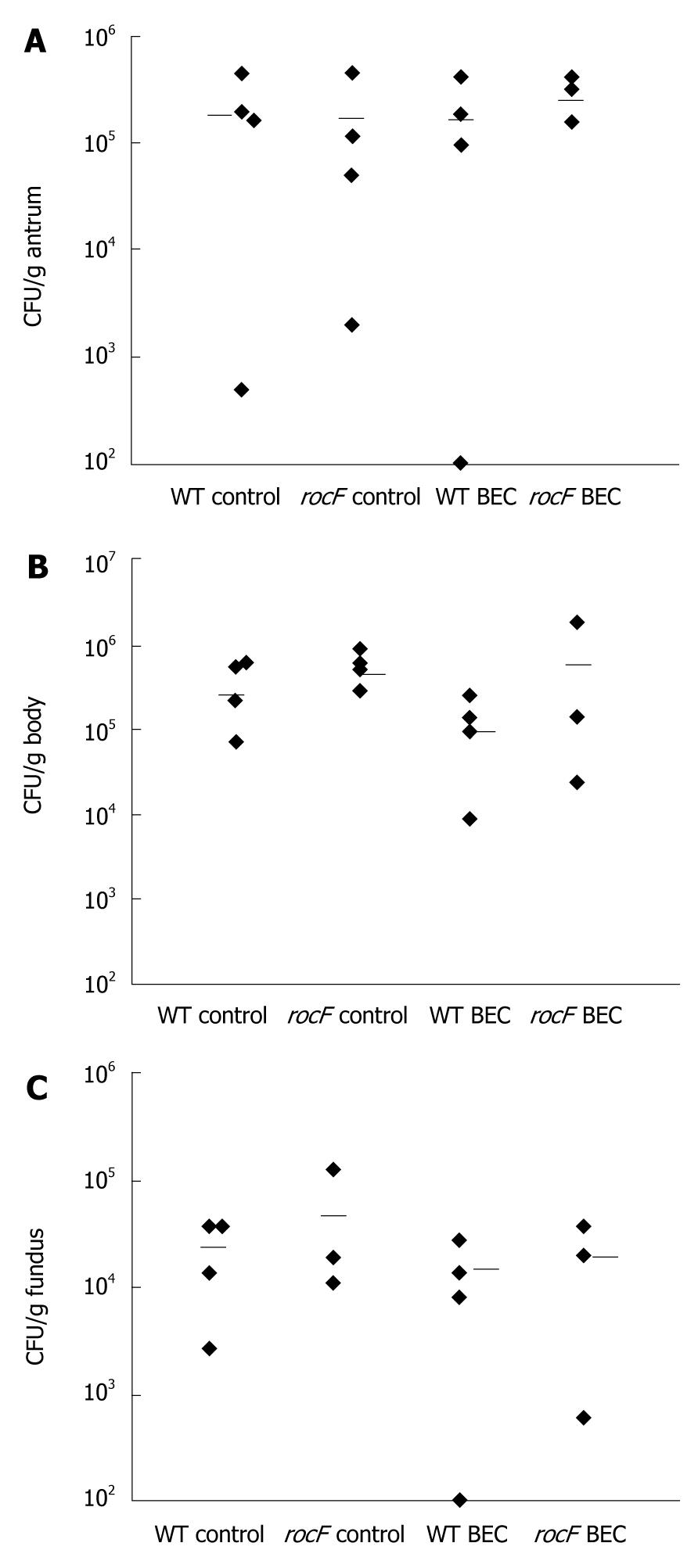
Figure 7 The rocF mutant of Helicobacter pylori colonizes wild-type mice treated with the arginase inhibitor S-(2-boronoethyl)-L-cysteine.
Wild-type (C57 BL/6) mice (n = 3 or 4 per group) were orally fed water or S-(2-boronoethyl)-L-cysteine (BEC) (50 mmol/L) once or twice daily for 3 d before oral inoculation with wild-type or rocF mutant Helicobacter pylori (H. pylori) strain SS1 (~108 CFU). The BEC animal groups continued to receive BEC daily until animals were euthanized. At 1 wk post-infection, animals were euthanized and the stomach dissected into antrum (A), body (B), or fundus (C). Limit of detection was ~100 CFU/g stomach and all animals that lacked H. pylori were set to this detection limit. Bar, mean CFU/g tissue. Usually each symbol represents one mouse. In some cases there are two mice represented by a symbol if the data overlapped.
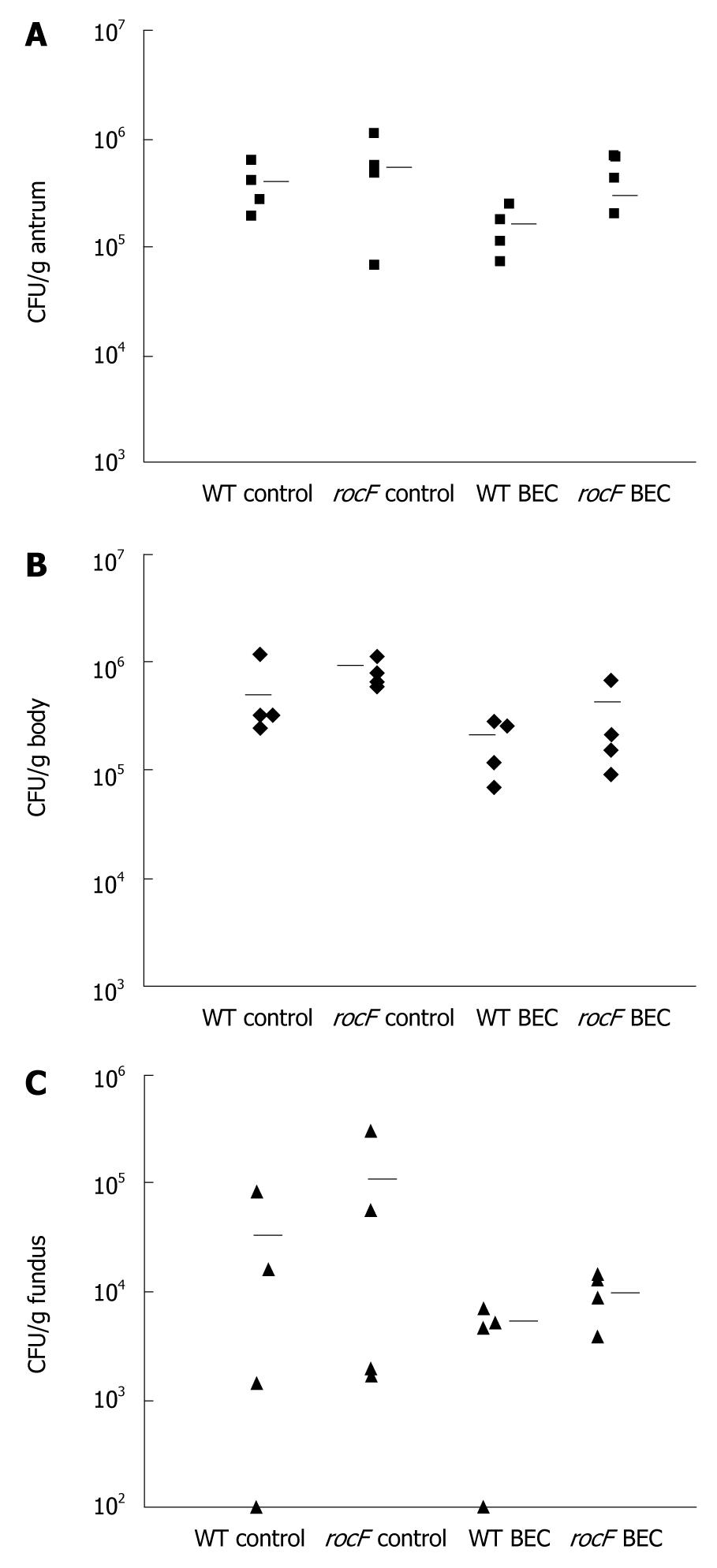
Figure 8 The rocF mutant of Helicobacter pylori colonizes arginase II knockout mice treated with the arginase inhibitor S-(2-boronoethyl)-L-cysteine.
Arginase II knockout mice (n = 3 or 4 per group) were orally fed water or S-(2-boronoethyl)-L-cysteine (BEC) (50 mmol/L) once or twice daily for 3 d before oral inoculation with wild-type or rocF mutant Helicobacter pylori strain SS1 (~108 CFU). The BEC animal groups continued to receive BEC daily until animals were euthanized. At 1 wk post-infection, animals were euthanized and the stomach dissected into antrum (A), body (B), or fundus (C). Limit of detection: ~100 CFU/g tissue. Bar, mean CFU/g stomach.
-
Citation: Kim SH, Langford ML, Boucher JL, Testerman TL, McGee DJ.
Helicobacter pylori arginase mutant colonizes arginase II knockout mice. World J Gastroenterol 2011; 17(28): 3300-3309 - URL: https://www.wjgnet.com/1007-9327/full/v17/i28/3300.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i28.3300