Copyright
©2007 Baishideng Publishing Group Co.
World J Gastroenterol. Jan 7, 2007; 13(1): 65-73
Published online Jan 7, 2007. doi: 10.3748/wjg.v13.i1.65
Published online Jan 7, 2007. doi: 10.3748/wjg.v13.i1.65
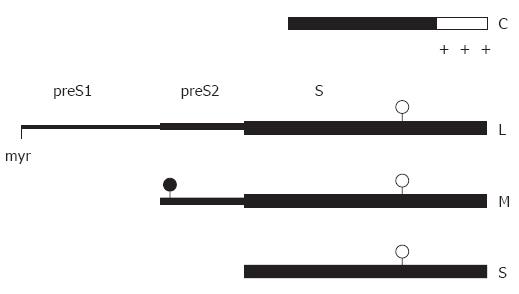
Figure 1 Linear map of the main structural HBV proteins.
The C-terminal region (open box) of the capsid protein C is rich in arginine residues (+). The sequence of the small envelope S is also present at the C termini of the M and L protein. The two larger envelope proteins contain the additional N-terminal preS2 and preS2 + preS1 domain, respectively. The L protein is myristylated at glycin 2 (myr), the preS2 domain of M is N-glycosylated (filled circles), and the S domain of all 3 proteins is partially N-glycosylated (open circles).
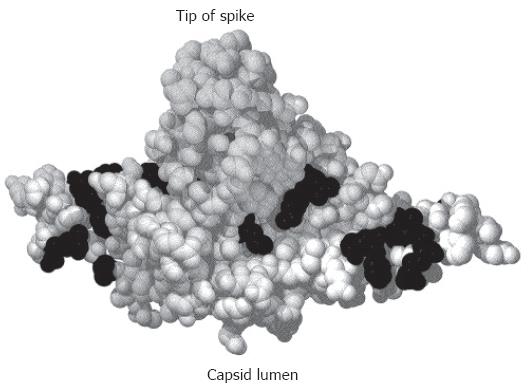
Figure 2 Crystal structure of a C-terminally truncated C protein dimer[21].
The spike protrudes upwards. The lumen of the capsid would be below the figure. Mutational analysis identified aa (shown in black) where the mutation was compatible with capsid formation and viral DNA synthesis in the lumen of the particle but blocked nucleocapsid envelopment[121].
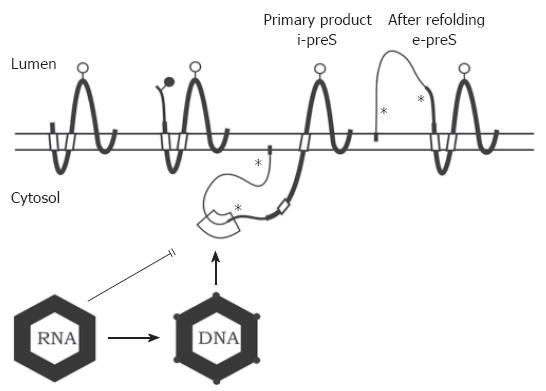
Figure 3 Transmembrane topology of the HBV envelope proteins and model for envelope-capsid interaction.
The transmembrane folding of the S protein is determined by an N-terminal and an internal signal shown as open boxes. The C-terminal domain is hydrophobic and probably embedded in the lipid bilayer (horizontal open bar). The C terminus is oriented towards the ER lumen. The folding of the M protein is similar to S. The preS2 domain of M (thinner line) is located in the ER lumen. In the initial folding of the L protein, the preS domains are located in the cytosol (i-preS). Whether the N-terminal myristate group (filled box) is inserted into the membrane as shown here is unknown. After refolding approximately half of the L chains expose the preS domains at the luminal side of the membrane (e-preS). Open and filled circles: see Figure 1. Asterisks indicate potential but unused N-glycosylation sites in preS of L. A domain in i-preS (boxed area) and in the cytosolic loop of S may interact with the capsid during budding. Immature capsids containing pregenomic RNA are not capable to bud. During viral DNA synthesis, the capsid shell changes (indicated by filled circles at the edges) and becomes competent for envelopment.
- Citation: Bruss V. Hepatitis B virus morphogenesis. World J Gastroenterol 2007; 13(1): 65-73
- URL: https://www.wjgnet.com/1007-9327/full/v13/i1/65.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i1.65