Copyright
©The Author(s) 2004.
World J Gastroenterol. Jul 15, 2004; 10(14): 2029-2033
Published online Jul 15, 2004. doi: 10.3748/wjg.v10.i14.2029
Published online Jul 15, 2004. doi: 10.3748/wjg.v10.i14.2029
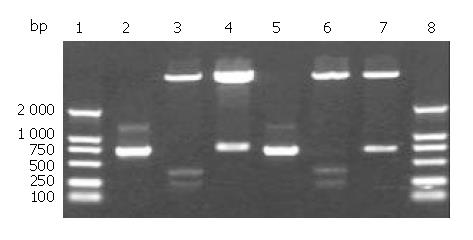
Figure 1 Analysis of recombinant non-fusion expression vectors pET32a/cFd and pET32a/cL digested by restrictive endonucleases.
1: DNA marker; 2: cFd; 3: pET32a/Nde I + Sal I; 4: pET32a/cFd/Nde I + Sal I; 5: cL; 6: pET32a/Nde I + Xho I; 7: pET32a/cL/Nde I + Xho I; 8: DNA marker.
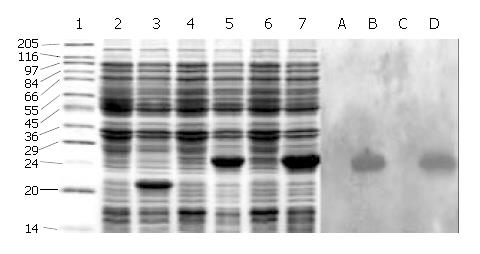
Figure 2 SDS-PAGE and Western blotting of the expressed products of cFd and cL.
1: High molecular mass marker; 2: Uninduced pET32a; 3: Induced pET32a; 4: Uninduced pET32a/cFd; 5: Induced pET32a/cFd; 6: Uninduced pET32a/cL; 7: Induced pET32a/cL; A: Uninduced pET32a/cFd; B: Induced pET32a/cFd; C: Uninduced pET32a/cL; D: Induced pET32a/cL.
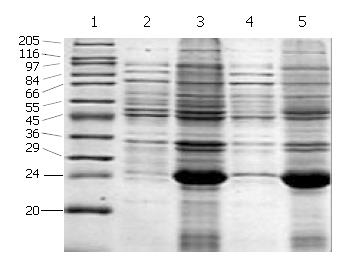
Figure 3 Location analysis of cFd and cL expression products on SDS-PAGE.
1: High molecular mass marker; 2: Supernatant of induced pET32a/cFd; 3: Inclusion body of induced pET32a/cFd; 4: Supernatant of induced pET32a/cL; 5: Inclusion body of induced pET32a/cL.
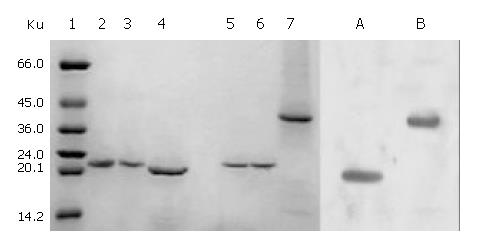
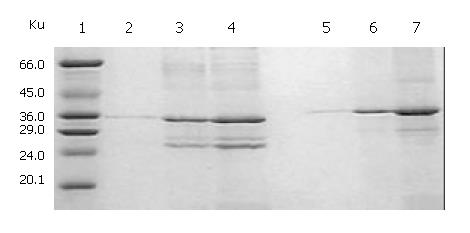
Figure 4 SDS-PAGE and Western blotting of renaturation of cFab antibody.
1: High molecular mass marker; 2: Reduced cFd; 3: Reduced cL; 4: Reduced cFab; 5: Unreduced cFd; 6: Un-reduced cL; 7: Unreduced cFab; A: Western blotting of reduced cFab; B: Western blotting of unreduced cFab.
Figure 5 Relationship between the starting total protein con-centration and denaturation analyzed by SDS-PAGE.
1: High molecular mass marker; 2, 3, 4: Precipitated proteins under reduced condition at starting total protein concentrations of 100, 200, 400 µg/mL, respectively; 5, 6, 7: Precipitated proteins under unreduced condition at starting total protein concentra-tions of 100, 200, 400 µg/mL, respectively.
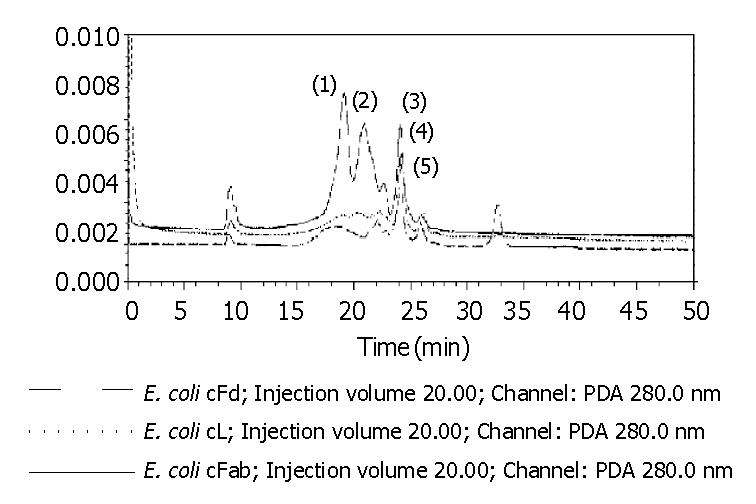
Figure 6 HPLC of cFd, cL and cFab after renaturation.
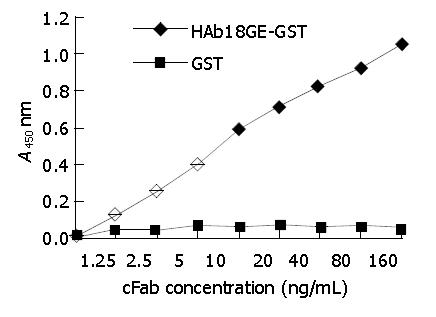
Figure 7 Detection of antigen-binding specificity of cFab by indirect ELISA.
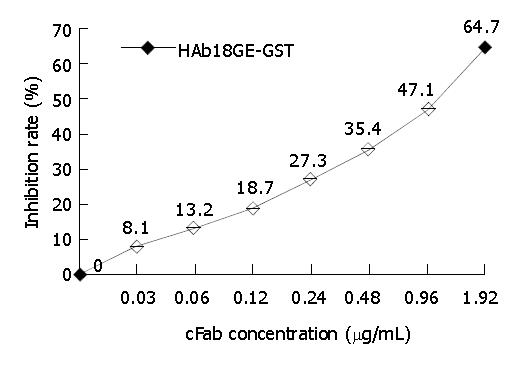
Figure 8 Detection of antigen-binding activity of cFab and HAb18 by competitive ELISA.
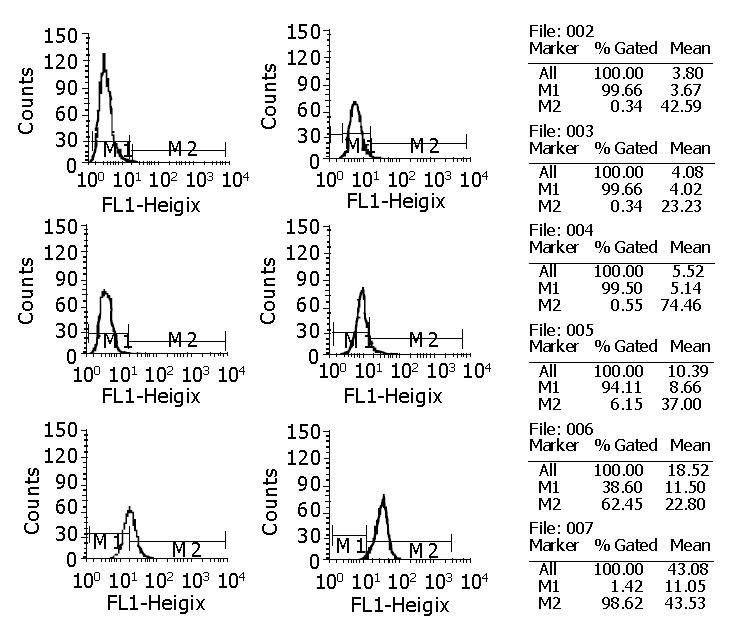
Figure 9 Detection of antigen-binding activity of cFab by FACS.
002: Negative control; 003: 0.008 µg/mL; 004: 0.04 µg/mL; 005: 0.2 µg/mL; 006: 1 µg/mL; 007: 5 µg/mL.
- Citation: Xing JL, Yang XM, Yao XY, Song F, Chen ZN. Prokaryotic expression and renaturation of engineering chimeric Fab antibody against human hepatoma. World J Gastroenterol 2004; 10(14): 2029-2033
- URL: https://www.wjgnet.com/1007-9327/full/v10/i14/2029.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i14.2029