Copyright
©The Author(s) 2025.
World J Methodol. Mar 20, 2025; 15(1): 95857
Published online Mar 20, 2025. doi: 10.5662/wjm.v15.i1.95857
Published online Mar 20, 2025. doi: 10.5662/wjm.v15.i1.95857
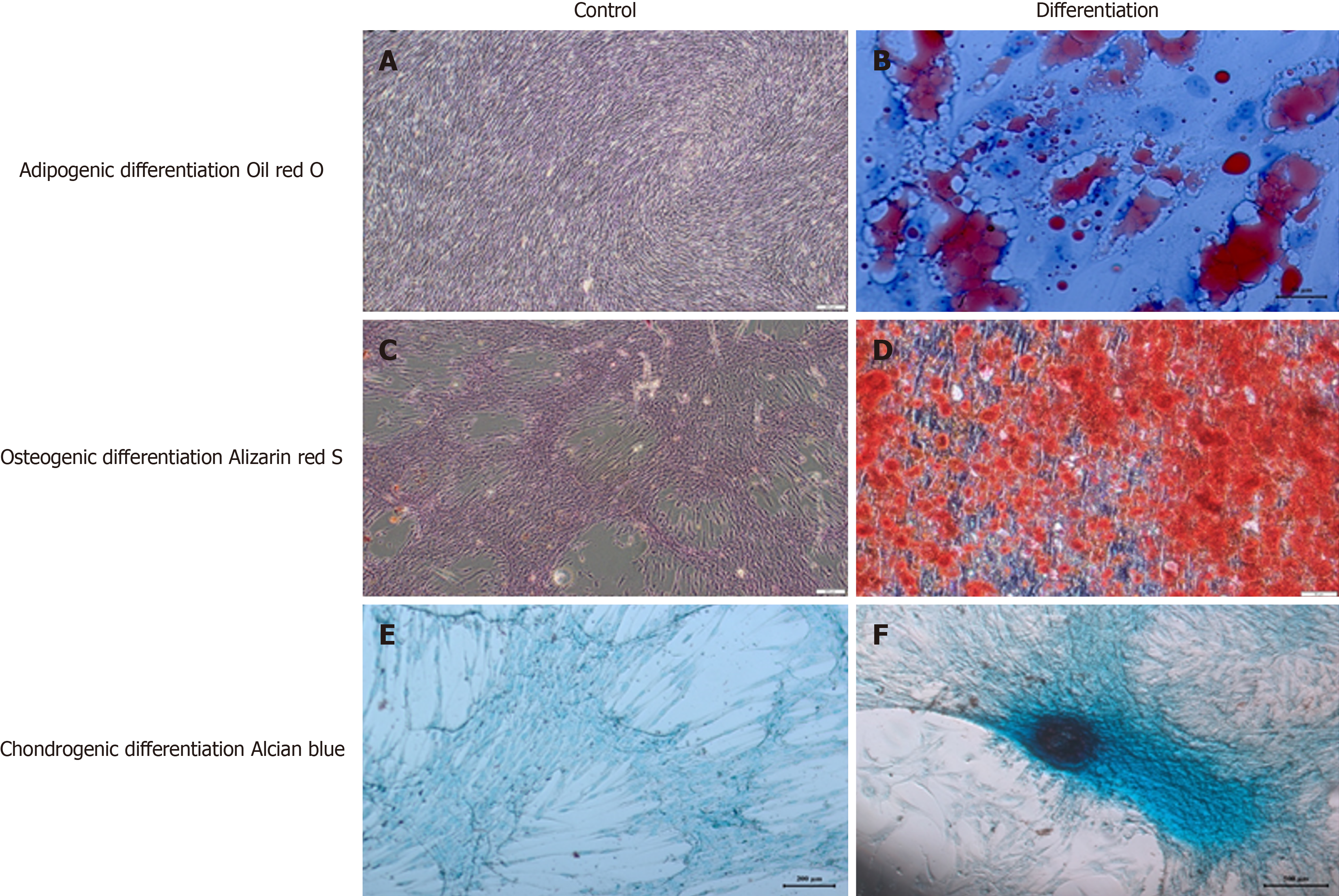
Figure 2 Detection of the differentiation potential of Wharton’s jelly-derived mesenchymal stem cells.
A and B: Wharton’s jelly-derived mesenchymal stem cells (WJ-MSCs) were cultured without adipogenic induction and cultured for 3 weeks in adipogenic differentiation medium. Adipogenic differentiation was evidenced by the formation of lipid vacuoles with oil red O staining; C and D: WJ-MSCs were cultured without osteogenic induction and cultured for 3 weeks in osteogenic differentiation medium. Osteogenic differentiation was evidenced by the detection of calcium deposits with Alizarin Red staining; E and F: WJ-MSCs were cultured without chondrogenic induction and cultured for 3 weeks in chondrogenic differentiation medium. Chondrogenic differentiation was evidenced with Alcian Blue staining[8]. Citation: Boyalı O, Kabatas S, Civelek E, Ozdemir O, Bahar-Ozdemir Y, Kaplan N, Savrunlu EC, Karaöz E. Allogeneic mesenchymal stem cells may be a viable treatment modality in cerebral palsy. World J Clin Cases 2024; 12: 1585-1596. Copyright © The Authors 2024. Published by Baishideng Publishing Group Inc.
- Citation: Kabatas S, Civelek E, Savrunlu EC, Karaaslan U, Yıldız Ö, Karaöz E. Advances in the treatment of autism spectrum disorder: Wharton jelly mesenchymal stem cell transplantation. World J Methodol 2025; 15(1): 95857
- URL: https://www.wjgnet.com/2222-0682/full/v15/i1/95857.htm
- DOI: https://dx.doi.org/10.5662/wjm.v15.i1.95857