Copyright
©The Author(s) 2017.
World J Nephrol. Jul 6, 2017; 6(4): 188-200
Published online Jul 6, 2017. doi: 10.5527/wjn.v6.i4.188
Published online Jul 6, 2017. doi: 10.5527/wjn.v6.i4.188
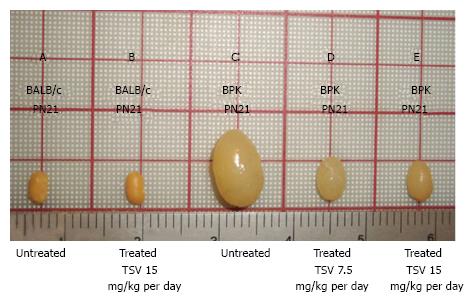
Figure 1 Tesevatinib treatment in the BPK model of autosomal recessive polycystic kidney disease.
Compared to wild-type BALB/c control kidneys (A) at PN21, BPK cystic kidneys (C) are extremely enlarged by PN21. TSV treatment resulted in a dose-dependent reduction in the overall kidney size at doses of 7.5 mg/kg per day (C) and 15 mg/kg per day (D) when compared to PN21 BPK untreated cystic kidneys (C). Treatment of wildtype BALB/c with 15 mg/kg per day (B) did not result in significant reduction of overall kidney size when compared to untreated BALB/c kidneys (A). This correlates directly with the total kidney weight (TKW) of each group listed in Table 1. Further, the morphology of wildtype TVS treated kidneys seen in Figure 2A shows no obvious signs of toxicity (Treatment interval: PN4-PN21). TSV: Tesevatinib.
- Citation: Sweeney WE, Frost P, Avner ED. Tesevatinib ameliorates progression of polycystic kidney disease in rodent models of autosomal recessive polycystic kidney disease. World J Nephrol 2017; 6(4): 188-200
- URL: https://www.wjgnet.com/2220-6124/full/v6/i4/188.htm
- DOI: https://dx.doi.org/10.5527/wjn.v6.i4.188