Published online Apr 19, 2024. doi: 10.5498/wjp.v14.i4.523
Peer-review started: November 21, 2023
First decision: January 24, 2024
Revised: February 7, 2024
Accepted: March 22, 2024
Article in press: March 22, 2024
Published online: April 19, 2024
Processing time: 147 Days and 15.2 Hours
Prior studies have noted great variability in the plasma levels of risperidone (RIS). Plasma concentrations of RIS and its active moiety are highly variable and depend on absorption, metabolism, and other predictors of metabolic dysregulation; however, these factors are poorly understood and the association between metabolic change and change in psychopathology is uncertain.
To ascertain the characteristics of chronic schizophrenic patients treated with RIS, and to assess their relationship with plasma RIS levels.
This was a descriptive cross-sectional study of 50 patients with a diagnosis of schizophrenic psychosis treated with RIS in a psychiatric service. The plasma concentrations of RIS and its metabolite 9-hydroxyrisperidone were determined by high performance liquid chromatography. The patients’ demographic and clinical characteristics, and psychopathologies were assessed, and the associations between clinical variables and plasma levels of RIS were explored.
Male patients received higher doses of RIS than female ones, but plasma concentrations of RIS and risperidone + 9-hydroxyrisperidone (active moiety) were higher in female patients. Age and the mean scores of the general psychopathology subscale of the Positive and Negative Syndrome Scale (PANSS) were significantly positively correlated with plasma concentrations of risperidone + 9-hydroxyrisperidone adjusted for weight and dose in all 50 subjects. In male subjects, we found a statistically significant positive correlation between the concentrations of risperidone + 9-hydroxyrisperidone in plasma/(dose × kg) and age, mean PANSS negative subscale scores, mean PANSS general psychopathology subscale scores, and mean PANSS total scores.
Long-term use of RIS should be closely monitored in older patients and females to minimize the risk of high concentrations which could induce side effects.
Core Tip: Prior studies have noted great variability in the plasma levels of risperidone (RIS). Fifty patients confirmed to have schizophrenia were selected for this study. We assessed the patients’ demographic and clinical characteristic, and psychopathologies, and explored the associations and correlations between clinical variables and plasma levels of RIS. The results of this study indicate that the long-term use of RIS should be closely monitored in older patients and females to minimize the risk of high concentrations which could induce side effects.
- Citation: Xu JW, Guan XB, Wang XY, Feng Y, Zhang Q, Zhu JJ, Chen JH. Relationship between plasma risperidone concentrations and clinical features in chronic schizophrenic patients in China. World J Psychiatry 2024; 14(4): 523-532
- URL: https://www.wjgnet.com/2220-3206/full/v14/i4/523.htm
- DOI: https://dx.doi.org/10.5498/wjp.v14.i4.523
Schizophrenia is a severe disabling psychiatric disorder which is found in all regions of the world; however, the etio
RIS is fundamentally metabolized by the hepatic microsomal enzyme cytochrome P450 (CYP)2D6, and, to a lesser extent, by CYP3A4[2]. Its main metabolite, 9-hydroxyrisperidone, is pharmacologically active. Preclinical studies have indicated that 9-hydroxyrisperidone has approximately 70% of the pharmacological activity of RIS[3]. Since the pharmacological activity of 9-hydroxyrisperidone is claimed to be similar to that of the parent compound, the sum of the plasma concentrations of RIS and 9-hydroxyrisperidone is referred to as the clinically relevant “active moiety”[4].
Large intra- and inter-individual variations in plasma concentrations of both RIS and 9-hydroxyrisperidone have been identified in prior studies[2]. Therapeutic drug monitoring (TDM) in the clinic uses the quantification of drug concentrations in plasma or serum to assist physicians in making treatment decisions related to an individual patient. The determination of plasma concentrations of RIS as well as 9-hydroxyrisperidone is used to evaluate patient compliance with the therapy, to optimize treatment, and to minimize the risk of adverse drug reactions (ADRs). By adjusting the dose, a drug concentration associated with the highest probability of response and the lowest risk of ADRs and toxic effects can be achieved. The TDM thus provides a valid method for individual dose titration and careful monitoring, and is strongly recommended in the guidelines for adults treated with RIS[5].
Plasma concentrations of RIS and active moiety are highly variable and depend on absorption, and metabolism, as well as other predictors (for example, age, sex, body mass index, and smoke) of metabolic dysregulation; however, these factors are poorly understood and the association between metabolic change and change in psychopathology is uncertain[6]. Therefore, these factors should be considered in studies.
The primary aim of the present study was to assess the plasma concentrations obtained at different daily doses for the commonly used drug RIS in a natural setting, to examine the clinical situation of patients with chronic schizophrenia treated with RIS, and the possible relations between patient characteristics and plasma concentrations of RIS.
This was a descriptive transversal study of all the patients treated with RIS in a Psychiatric Hospital with the diagnosis of schizophrenic psychosis. Fifty patients confirmed to have schizophrenia by a group of psychiatrists according to the ICD-10 were selected for this study. None of the patients had serious illness, or current alcohol and/or drug abuse. Patients were treated with oral RIS at doses ranging from 2 to 6 mg/d. The RIS dose was adjusted individually according to the clinical response. The plasma concentrations of RIS and its metabolite 9-hydroxyrisperidone were determined by high performance liquid chromatography (HPLC). Sociodemographic and clinical variables were studied, together with anthropometric measurements, life signs, hemogram, metabolic parameters, and ADRs, between February and March 2021.
HPLC uses a high-pressure infusion pump to pump the specified mobile phase into a chromatographic column containing fillers; the injected sample is brought into the chromatographic column by the mobile phase, and each component is subjected to intermolecular forces in the column. The adsorption-desorption process is carried out between the mobile phase and the stationary phase, so that each component is separated and enters the detector for detecting. A chromatographic signal is recorded and processed by the integrating instrument or the data processing system. In the daily routine testing, the samples are tested in parallel with quality controls, and three quality control concentration levels are used to observe the passing of quality controls. The standard curve graph is prepared, and the data in this study are all between the detectable range of each drug concentration. Chromatographic conditions were as follows: One-dimensional column: AstonSX1 (3.5 mm × 25 mm, 5 μm); intermediate column: Aston SCB (3.5 mm × 10 mm, 5 μm); two-dimensional column: Aston SCB (4.6 mm × 125 mm, 5 μm). The steps of RIS and paliperidone (major metabolite of RIS) detection were: Processing method: ORG-1 1000 μL + blood sample 400 μL, high-speed centrifugation to take the supernatant; detection wavelength: CH1: 276 nm, CH2: 286 nm; flow rate: Pump A: 1.20 mL/min, pump B: 0.01 mL/min, pump C: 0.80 mL/min; temperature: 40 °C; injection volume: 500 μL.
The clinical and research staff and participants were not blinded to any of the study conditions, as there was no comparison control group. Clinical interviews were conducted and blood was taken and sent for laboratory analysis. The following parameters were evaluated: An electrocardiogram was performed to evaluate patients’ heart rate and QT interval (QTc). Data on patient age, weight, body mass index, blood pressure, and cigarettes smoked per day were also acquired. The discrete evaluated parameters included sex, smoker or not, and taking trihexyphenidyl/laxatives or not (according to the doctor's advice in the medical record) as ADRs occurred. Plasma concentrations of RIS and 9-hydroxyrisperidone were determined while fasting in the morning, without having eaten during the night or taken the breakfast dose of RIS. Using this value, the plasma concentrations of “active moiety” (RIS + 9-hydroxyrisperidone) and concentrations of RIS + 9-hydroxyrisperidone in plasma/(dose × kg) were calculated.
Psychopathological examination, which was completed within 3 d of blood testing, covered the following areas: Psychotic symptoms were assessed by means of the Positive and Negative Syndrome Scale (PANSS)[7]; depressive symptoms were scored on the Patient Health Questionnaire Depression Scale (PHQ-9)[8], with the results classified as follows: 0-4 points, normal; 5-9 points, mild depression; 10-14 points, moderate depression; and 15-27 points, severe depression.
All statistical analyses were carried out using IBM SPSS statistics, version 22.0 and GraphPad PRISM, version 7.0. The categorical variables are described as frequencies and percentages, while continuous variables are reported as the mean ± SD or range. Parameters were tested for normal distribution by the one-sample Kolmogorov-Smirnoff test. In the case of continuous variables, the Student's t-test was used to compare differences between the averages among groups for two independent samples of normally distributed data, or the Mann-Whitney U-test was used to compare data which was not normally distributed. The Pearson correlation coefficient was computed for normally distributed data, and the Spearman rank correlation coefficient was computed for non-normally distributed data. A P value < 0.05 was considered statistically significant.
A total of 52 patients diagnosed with schizophrenia and treated with RIS were initially enrolled. Of these 52 patients at the time of the study, 2 were excluded for the following reasons: One id not speak and was unable to complete the scales measurement, and the other because of the absence of plasma concentration of RIS. In the end, 50 subjects were included in the study.
The patients were aged from 38 to 69 years old (mean age, 58.4 years, SD = 8.3); 36% (n = 18) were women. All patients had been diagnosed with schizophrenia. Of these, 90% had been in treatment with RIS for more than 5 years, and none had undergone dose changes during the 2 mo prior to the study. Seven (14%) were obese [body mass index (BMI) ≥ 30], while 70% had normal weight (BMI < 25). All smokers (n = 23; 46%) were male, with an average consumption of 16.1 cigarettes/d (SD = 8.8). In the depressive symptom evaluation, three patients (6.0%) showed mild depression, while the others scored within the normal range according to the PHQ-9 criteria. Details are displayed in Table 1.
| Characteristic (n = 50) | Minimum value | Maximum value | Mean value | SD |
| Age (yr) | 38 | 69 | 58.38 | 8.31 |
| BMI | 16.02 | 36.05 | 23.95 | 4.82 |
| Course of illness (yr) | 15 | 51 | 33.32 | 9.18 |
| Systolic blood pressure (mmHg) | 90 | 180 | 130.08 | 18.21 |
| Diastolic blood pressure (mmHg) | 60 | 105 | 78.60 | 10.32 |
| Heart rate (per min) | 60 | 103 | 79.86 | 9.79 |
| QTc | 0.38 | 0.52 | 0.45 | 0.03 |
| Mean PANSS positive subscale score | 7 | 20 | 10.92 | 3.17 |
| Mean PANSS negative subscale score | 9 | 31 | 22.32 | 4.20 |
| Mean PANSS general psychopathology subscale score | 22 | 51 | 31.04 | 5.07 |
| Mean PANSS total score | 48 | 101 | 64.28 | 8.89 |
| PHQ-9 | 0 | 9 | 1.82 | 1.64 |
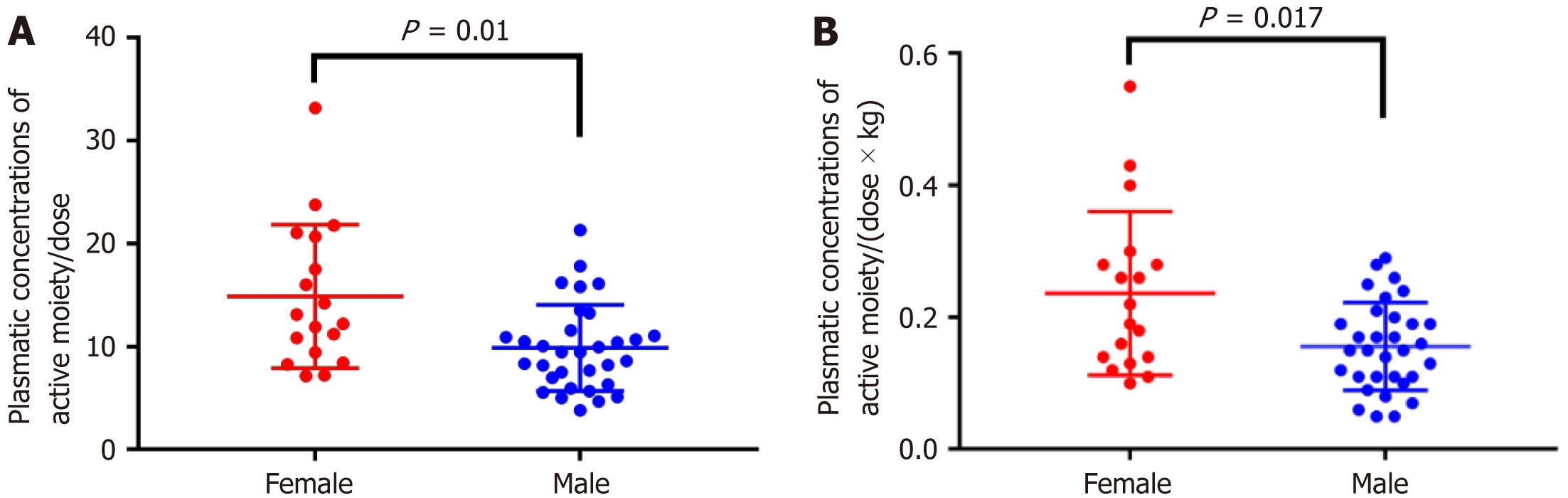
The doses of RIS prescribed varied from 2 mg/d to a maximum dose of 6 mg/d, and plasma concentrations of RIS varied from 0 to 43.68 ng/mL, while plasma concentrations of 9-hydroxyrisperidone varied from 10.57 to 98.87 ng/mL. Table 2 shows the dose of RIS and plasma concentrations of RIS and 9-hydroxyrisperidone divided according to gender. Male subjects received higher doses of RIS than female ones, in terms of absolute dose and dose/kg. However, plasma concentrations of RIS and risperidon + 9-hydroxyrisperidone (active moiety) were higher in women, while plasm concentrations of 9-hydroxyrisperidone were higher in men, although these differences were not statistically significant. Statistically significant values in comparison of average plasma concentrations of risperidon + 9-hydroxyrisperidone/dose (Figure 1A) and (dose × kg) according to sex were found (Figure 1B).
| Sex | n | Average | SD | P value | |
| Risperidone dose (mg)1 | Male | 32 | 4.09 | 1.12 | 0.003 |
| Female | 18 | 3.11 | 0.90 | ||
| Total | 50 | 3.74 | 1.14 | ||
| Risperidone dose per kg weight2 | Male | 32 | 0.06 | 0.02 | 0.010 |
| Female | 18 | 0.05 | 0.02 | ||
| Total | 50 | 0.06 | 0.02 | ||
| Concentration of risperidone in plasma (ng/mL)1 | Male | 32 | 8.61 | 10.80 | 0.134 |
| Female | 18 | 15.13 | 15.27 | ||
| Total | 50 | 10.96 | 12.83 | ||
| Concentration of 9-hydroxyrisperidone in plasma (ng/mL)1 | Male | 32 | 32.37 | 18.63 | 0.887 |
| Female | 18 | 29.24 | 10.25 | ||
| Total | 50 | 31.24 | 16.07 | ||
| Concentration of risperidone + 9-hydroxyrisperidone in plasma (ng/mL)1 | Male | 32 | 40.98 | 23.98 | 0.293 |
| Female | 18 | 44.37 | 18.79 | ||
| Total | 50 | 42.20 | 22.11 | ||
| Concentration of risperidone + 9-hydroxyrisperidone in plasma/dose2 | Male | 32 | 9.87 | 4.18 | 0.010 |
| Female | 18 | 14.89 | 6.96 | ||
| Total | 50 | 11.68 | 5.81 | ||
| Concentration of risperidone + 9-hydroxyrisperidone in plasma/(dose × kg)1 | Male | 32 | 0.16 | 0.07 | 0.017 |
| Female | 18 | 0.24 | 0.12 | ||
| Total | 50 | 0.18 | 0.10 |
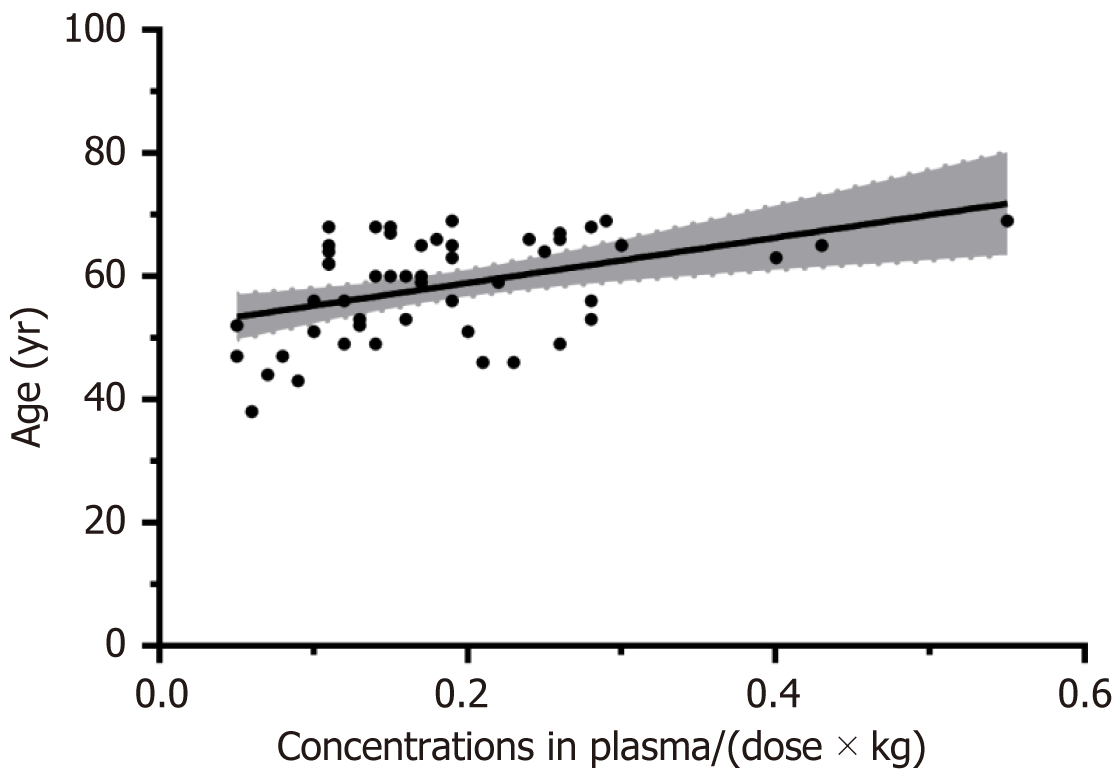
Regarding the clinical and psychopathological variables, we did not find significant associations between the concentrations of risperidon + 9-hydroxyrisperidone in plasma/(dose × kg) and any of the variables studied, except for age (P = 0.015, Figure 2) and mean PANSS general psychopathology subscale scores (P = 0.027), which were significantly positively correlated with plasma concentrations of active moiety adjusted for weight and dose in the 50 subjects.
In male subjects (n = 32), a statistically significant positive correlation was found between the concentrations of risperidon + 9-hydroxyrisperidone in plasma/(dose × kg) and age (P < 0.05), mean PANSS negative subscale scores (P < 0.05), mean PANSS general psychopathology subscale scores (P < 0.05), and mean PANSS total scores (P < 0.05). Regarding female subjects (n = 18), no correlation was found between the average values of plasmatic risperidon + 9-hydroxyrisperidone/(dose × kg) and the clinical and psychopathological variables (P > 0.05, Table 3).
| Correlation of average concentration in plasma/(dose × kg) with | Correlation coefficient | P value |
| Male (n = 32) | ||
| Age (yr) | 0.4572 | 0.008 |
| Number of cigarettes (smokers) | -0.3591 | 0.092 |
| Systolic blood pressure | 0.0962 | 0.600 |
| Diastolic blood pressure | 0.1262 | 0.492 |
| BMI | -0.2711 | 0.134 |
| Heart rate | -0.1731 | 0.344 |
| QTc | 0.0602 | 0.743 |
| Mean PANSS positive scale score | 0.1792 | 0.328 |
| Mean PANSS negative scale score | 0.3731 | 0.035 |
| Mean PANSS general psychopathology subscale score | 0.3891 | 0.028 |
| Mean PANSS total score | 0.4812 | 0.005 |
| Female (n = 18) | ||
| Age | 0.4361 | 0.071 |
| Systolic blood pressure | -0.1732 | 0.492 |
| Diastolic blood pressure | 0.0242 | 0.925 |
| BMI | -0.4462 | 0.064 |
| Heart rate | 0.1322 | 0.600 |
| QTc | 0.0592 | 0.816 |
| Mean PANSS positive subscale score | -0.2541 | 0.310 |
| Mean PANSS negative subscale score | -0.1302 | 0.607 |
| Mean PANSS general psychopathology subscale score | 0.3752 | 0.125 |
| Mean PANSS total score | 0.0642 | 0.801 |
Regarding ADRs, anticholinergic or laxative medications were taken according to the doctor’s recommendations; such a prescription was indicative of an ADR. Ten of the 50 patients were taking trihexyphenidyl (anticholinergic drug), and there was no statistical difference in each plasma drug concentration variable when compared between the ADR and non-ADR groups (P < 0.05). Among the 50 patients, 10 were taking laxative drugs, and no statistical difference was found in all variables in group comparison (P < 0.05) (Table 4).
| Trihexyphenidyl group (n = 10) | No trihexyphenidyl group (n = 40) | P/Z value | |
| Risperidone dose per kg weight2 | 0.07 ± 0.03 | 0.06 ± 0.02 | 0.129 |
| Concentration of risperidone in plasma (ng/mL)1 | 11.18 ± 11.98 | 10.90 ± 13.18 | 0.899 |
| Concentration of 9-hydroxyrisperidone in plasma (ng/mL)2 | 42.94 ± 26.33 | 28.32 ± 10.98 | 0.117 |
| Concentration of risperidon + 9-hydroxyrisperidone in plasma (ng/mL)2 | 54.12 ± 33.68 | 39.22 ± 17.52 | 0.205 |
| Concentration of risperidon + 9-hydroxyrisperidone in plasma/dose1 | 11.77 ± 5.67 | 11.65 ± 5.92 | 0.923 |
| Concentration of risperidon + 9-hydroxyrisperidone in plasma/(dose × kg)1 | 0.17 ± 0.08 | 0.19 ± 0.10 | 0.577 |
| Laxative drug group (n = 10) | No laxative drug group (n = 40) | P/Z value | |
| Risperidone dose per kg weight2 | 0.06 ± 0.02 | 0.06 ± 0.02 | 0.667 |
| Concentration of risperidone in plasma (ng/mL)1 | 11.87 ± 14.22 | 10.71 ± 12.65 | 0.971 |
| Concentration of 9-hydroxyrisperidone in plasma (ng/mL)1 | 37.77 ± 28.34 | 29.61 ± 11.20 | 0.914 |
| Concentration of risperidon + 9-hydroxyrisperidone in plasma (ng/mL)1 | 49.64 ± 36.20 | 40.35 ± 17.15 | 0.877 |
| Concentration of risperidon + 9-hydroxyrisperidone in plasma/dose1 | 12.81 ± 6.25 | 11.39 ± 5.75 | 0.465 |
| Concentration of risperidon + 9-hydroxyrisperidone in plasma/(dose × kg)1 | 0.19 ± 0.07 | 0.18 ± 0.11 | 0.607 |
This study investigated the sociodemographic and clinical characteristics of 55 patients diagnosed with chronic schizophrenia disorder and treated with RIS, in order to clarify any possible associations between these variables and the dosage and plasma concentrations of RIS. Many patients in this study were using RIS for a long time to guarantee blood collections occurring after achieving steady plasma concentrations of RIS and 9-hydroxyrisperidone.
Based on previous studies of groups of patients with chronic schizophrenia, the characteristics of this cohort can be expected: Mostly male, with high rates of smoking, and mainly negative symptoms. However, the small sample size and male predominance could be considered as limitations of this work.
Our study findings show that age was positive correlated with concentrations of RIS + 9-hydroxyrisperidone in plasma/(dose × kg). Some smaller studies have reported slower elimination and/or higher levels of 9-hydroxyrisperidone in the elderly[9-11]. Elimination of 9-hydroxyrisperidone is mainly renal[12], and the most plausible expla
We found that plasma concentrations were significantly higher in women than in men for RIS + 9-hydroxyrisperidone adjusted for dose, as well as for active moiety adjusted for weight and dose, although the men received higher doses of RIS than the women. The same result has been observed in previous studies using SGA[13]. Several factors may explain these sex-related differences, including differences in hepatic clearance of drugs, caused by a lower liver volume in women, while the possible variations in compliance for antipsychotics between males and females should be taken into account[14], although the study was conducted in a hospital setting while all patients are hospitalized. As this was a monocentric study, men received higher doses than women; however, this could be a function of the predominance of men in the relatively small sample; as such, these results may not be generalizable to other settings.
Smoking prevalence in schizophrenic patients is higher than that in the general population[15]. In this study, smoking habits did not appear to influence the plasma concentrations of RIS and 9-hydroxyrisperidone. Berecz et al[16] reported that no influence of smoking on RIS metabolism could be found among 40 patients (Berecz et al[16] unpublished results). It should be noted that nicotine induces CYP1A2 and CYP2B6 activity, while RIS is extensively metabolized in the liver by CYP3A4 as well as CYP2D6 into the major active metabolite, 9-hydroxyrisperidone[17-19]. This metabolite is the predominant circulating molecule and appears to be of approximately equal efficacy as the parent compound[20]. This may explains why smoking has no influence on RIS metabolism.
The risk of cardiac side-effects by antipsychotic drugs has become a matter of public concern which can result in a prolongation of the QTc on the electrocardiogram[16]. RIS can increase the corrected QTc, although clinically relevant QTc prolongation is rare[21]. In this study, no correlation was found between weight and dose-adjusted concentrations of RIS active metabolite and QTc. This may indicate that RIS at a therapeutically effective plasma concentration does not seem to predispose patients to QTc interval lengthening. Nevertheless, this result has to be interpreted carefully due to the small sample size. One previous study reported that in patients treated with RIS, the QTc was related to CYP2D6 genotypes[22]; however, none of the patients were at risk of arrhythmia.
In our small sample, concentrations of active metabolite adjusted for weight and dose in the steady state were positively correlated with clinical scales, including mean PANSS negative subscale scores, mean PANSS general psychopathology subscale scores, and mean PANSS total scores only in males. We speculated that patients with a higher PANSS score may have more obvious psychiatric symptoms and thus may be prescribed a higher dose of RIS, leading to the higher plasma concentrations of RIS. One prior prospective study found no correlation between serum concentrations of RIS (including sum and ratio of RIS and 9-hydroxyrisperidone) and any other clinical values (e.g,. PANSS score)[23]. This prior study involved younger patients without any prior exposure to RIS as a prerequisite. Conversely, the present study was only a cross-sectional study with older subjects who had been taking RIS for a long time and had stable psychiatric symptoms. Therefore, the relationship between RIS concentrations (concentrations of active metabolite adjusted for weight and dose) and psychiatric symptoms in patients (especially among men) with chronic psychosis may need to be clarified with further follow-up.
We chose the use of anticholinergic drugs and laxatives as criteria for ADR, and found no difference between the ADR group and non-ADR group. Our findings are partly consistent with previous data supporting a prominent role of 9-hydroxyrisperidone, but not of RIS, in the development of ADRs[3]. RIS has a high 5-HT2A/D2 ratio, which should protect against extrapyramidal symptoms. However, at higher doses, RIS produces significant EPS, indicating that 5-HT2A antagonism alone cannot eliminate the EPS associated with substantial D2 receptor blockade[24]. In this study, we found that the concentration of 9-hydroxyrisperidone in the ADR group was higher than that of the non-ADR group, although this difference did not reach significance. The metabolite 9-hydroxyrisperidone seems to be the major circulating active moiety, with plasma concentrations 22-fold higher than those of RIS[25]. Clinicians may be advised to reduce the daily dosage in patients based upon the concentration of 9-hydroxyrisperidone rather than RIS. A further limitation of this study is the lack of an association between EPS and RIS levels. Similarly, we did not find a relationship in this sample between either BMI or blood glucose and plasma concentrations of RIS, which may be attributed to the smaller sample size and long-term RIS administration. As such, further studies with larger samples are needed to draw definite conclusions.
To conclude, the results of this study indicate that the long-term use of RIS should be closely monitored in older patients and females to minimize the risk of high concentrations which could induce side effects. The variability of the dose of RIS, as well as the physical and psychopathological situation of patients underlines the importance of therapeutic monitoring of plasma RIS and 9-hydroxyrisperidone concentrations to adjust the dose of RIS used in patients with chronic schizophrenia. These study findings provide useful insight to understand and address how TDM is necessary in schizophrenic patients receiving RIS while undergoing long-term hospitalization.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Chinese Association of Psychiatry; Basic and Clinical Branch of Psychiatry, Chinese Neuroscience Society; Mental Illness Professional Committee of Chinese Association of Integrated Traditional and Western Medicine; Professional Committee of Psychology and Psychiatry, Chinese Society of Research Hospitals.
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chakrabarti S, India; Pavón L, Mexico S-Editor: Qu XL L-Editor: Wang TQ P-Editor: Zhang YL
| 1. | Azizi E, Zavaran Hosseini A, Soudi S, Noorbala AA. Alteration of Serum Levels of Cytokines in Schizophrenic Patients before and after Treatment with Risperidone. Iran J Allergy Asthma Immunol. 2019;18:262-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Grundmann M, Kacirova I, Urinovska R. Therapeutic drug monitoring of atypical antipsychotic drugs. Acta Pharm. 2014;64:387-401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Schoretsanitis G, Stegmann B, Hiemke C, Gründer G, Schruers KR, Walther S, Lammertz SE, Haen E, Paulzen M. Pharmacokinetic patterns of risperidone-associated adverse drug reactions. Eur J Clin Pharmacol. 2016;72:1091-1098. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | van Beijsterveldt LE, Geerts RJ, Leysen JE, Megens AA, Van den Eynde HM, Meuldermans WE, Heykants JJ. Regional brain distribution of risperidone and its active metabolite 9-hydroxy-risperidone in the rat. Psychopharmacology (Berl). 1994;114:53-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 131] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Taurines R, Fekete S, Preuss-Wiedenhoff A, Warnke A, Wewetzer C, Plener P, Burger R, Gerlach M, Romanos M, Egberts KM. Therapeutic drug monitoring in children and adolescents with schizophrenia and other psychotic disorders using risperidone. J Neural Transm (Vienna). 2022;129:689-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 6. | Pillinger T, McCutcheon RA, Vano L, Mizuno Y, Arumuham A, Hindley G, Beck K, Natesan S, Efthimiou O, Cipriani A, Howes OD. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry. 2020;7:64-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 500] [Cited by in F6Publishing: 443] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 7. | Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13937] [Cited by in F6Publishing: 14755] [Article Influence: 398.8] [Reference Citation Analysis (0)] |
| 8. | Teymoori A, Gorbunova A, Haghish FE, Real R, Zeldovich M, Wu YJ, Polinder S, Asendorf T, Menon D; Center-Tbi Investigators And Participants; V Steinbüchel N. Factorial Structure and Validity of Depression (PHQ-9) and Anxiety (GAD-7) Scales after Traumatic Brain Injury. J Clin Med. 2020;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 9. | Aichhorn W, Weiss U, Marksteiner J, Kemmler G, Walch T, Zernig G, Stelzig-Schoeler R, Stuppaeck C, Geretsegger C. Influence of age and gender on risperidone plasma concentrations. J Psychopharmacol. 2005;19:395-401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Maxwell RA, Sweet RA, Mulsant BH, Rosen J, Kirshner MA, Kastango KB, Pollock BG. Risperidone and 9-hydroxyrisperidone concentrations are not dependent on age or creatinine clearance among elderly subjects. J Geriatr Psychiatry Neurol. 2002;15:77-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Molden E, Waade RB, Hoff M, Haslemo T. Impact of Ageing on Serum Concentrations of Risperidone and Its Active Metabolite in Patients with Known CYP2D6 Genotype. Basic Clin Pharmacol Toxicol. 2016;119:470-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | de Leon J, Wynn G, Sandson NB. The pharmacokinetics of paliperidone versus risperidone. Psychosomatics. 2010;51:80-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Jönsson AK, Spigset O, Reis M. A Compilation of Serum Concentrations of 12 Antipsychotic Drugs in a Therapeutic Drug Monitoring Setting. Ther Drug Monit. 2019;41:348-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 14. | Castberg I, Westin AA, Spigset O. Does level of care, sex, age, or choice of drug influence adherence to treatment with antipsychotics? J Clin Psychopharmacol. 2009;29:415-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Ohta C, Yasui-Furukori N, Furukori H, Tsuchimine S, Saito M, Nakagami T, Yoshizawa K, Kaneko S. The effect of smoking status on the plasma concentration of prolactin already elevated by risperidone treatment in schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:573-576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Berecz R, Dorado P, De La Rubia A, Cáceres MC, Degrell I, LLerena A. The role of cytochrome P450 enzymes in the metabolism of risperidone and its clinical relevance for drug interactions. Curr Drug Targets. 2004;5:573-579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Bork JA, Rogers T, Wedlund PJ, de Leon J. A pilot study on risperidone metabolism: the role of cytochromes P450 2D6 and 3A. J Clin Psychiatry. 1999;60:469-476. [PubMed] [Cited in This Article: ] |
| 18. | Fang J, Bourin M, Baker GB. Metabolism of risperidone to 9-hydroxyrisperidone by human cytochromes P450 2D6 and 3A4. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:147-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 181] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 19. | Alvarez-Herrera S, Escamilla R, Medina-Contreras O, Saracco R, Flores Y, Hurtado-Alvarado G, Maldonado-García JL, Becerril-Villanueva E, Pérez-Sánchez G, Pavón L. Immunoendocrine Peripheral Effects Induced by Atypical Antipsychotics. Front Endocrinol (Lausanne). 2020;11:195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Yoshimura R, Ueda N, Nakamura J. Possible relationship between combined plasma concentrations of risperidone plus 9-hydroxyrisperidone and extrapyramidal symptoms. Preliminary study. Neuropsychobiology. 2001;44:129-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Kloosterboer SM, de Winter BCM, Reichart CG, Kouijzer MEJ, de Kroon MMJ, van Daalen E, Ester WA, Rieken R, Dieleman GC, van Altena D, Bartelds B, van Schaik RHN, Nasserinejad K, Hillegers MHJ, van Gelder T, Dierckx B, Koch BCP. Risperidone plasma concentrations are associated with side effects and effectiveness in children and adolescents with autism spectrum disorder. Br J Clin Pharmacol. 2021;87:1069-1081. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 22. | Llerena A, Berecz R, Dorado P, de la Rubia A. QTc interval, CYP2D6 and CYP2C9 genotypes and risperidone plasma concentrations. J Psychopharmacol. 2004;18:189-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Lostia AM, Mazzarini L, Pacchiarotti I, Lionetto L, De Rossi P, Sanna L, Sani G, Kotzalidis GD, Girardi P, Simmaco M, Tatarelli R. Serum levels of risperidone and its metabolite, 9-hydroxyrisperidone: correlation between drug concentration and clinical response. Ther Drug Monit. 2009;31:475-481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Marder SR, Meibach RC. Risperidone in the treatment of schizophrenia. Am J Psychiatry. 1994;151:825-835. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 749] [Cited by in F6Publishing: 625] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 25. | Mauri MC, Paletta S, Di Pace C, Reggiori A, Cirnigliaro G, Valli I, Altamura AC. Clinical Pharmacokinetics of Atypical Antipsychotics: An Update. Clin Pharmacokinet. 2018;57:1493-1528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |