Copyright
©The Author(s) 2023.
World J Psychiatry. Aug 19, 2023; 13(8): 511-523
Published online Aug 19, 2023. doi: 10.5498/wjp.v13.i8.511
Published online Aug 19, 2023. doi: 10.5498/wjp.v13.i8.511
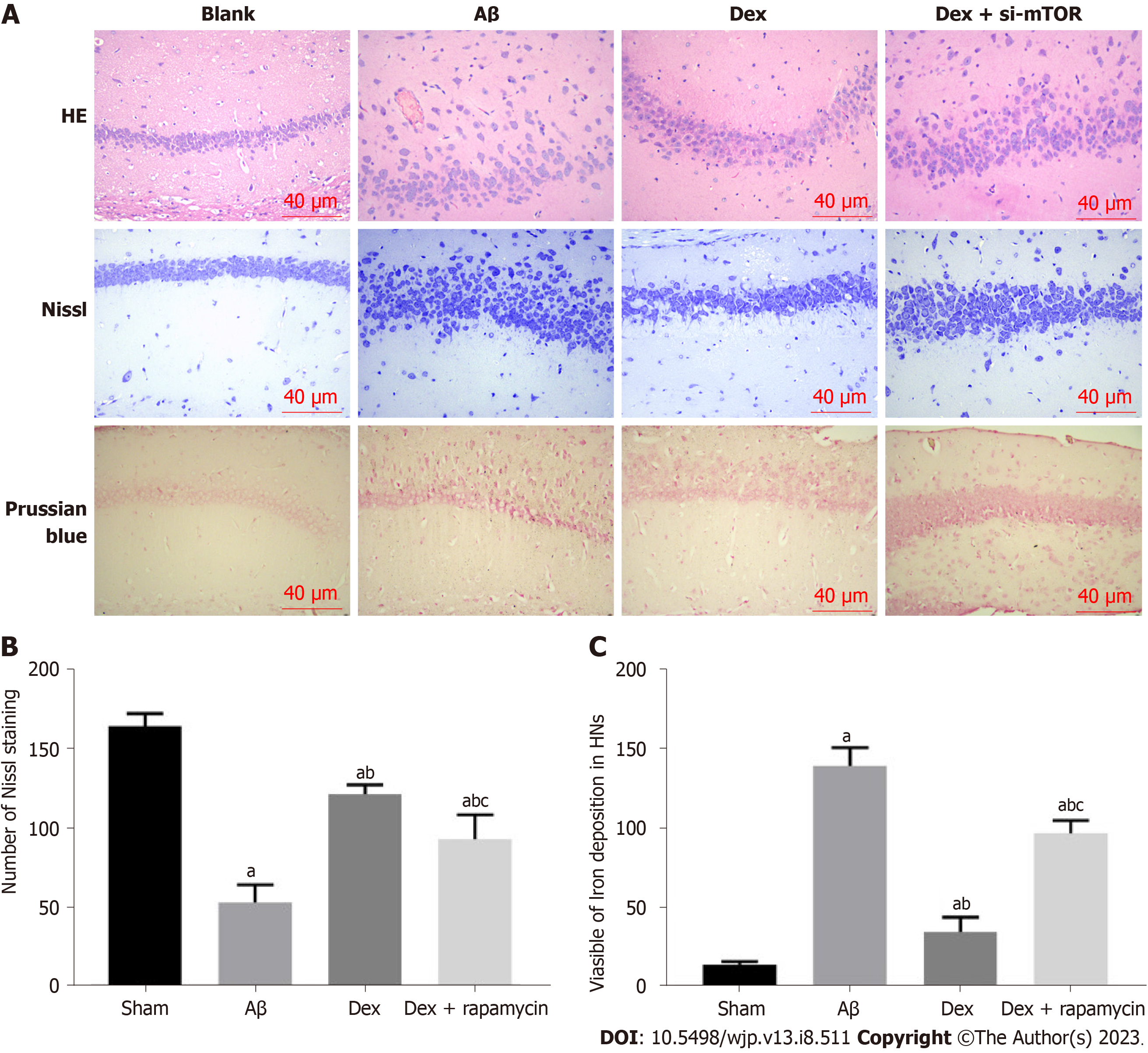
Figure 5 Comparison of pathological changes, nerve cell injury and iron deposition in mouse brain tissue in the different treatment groups.
A: Hematoxylin and eosin, Nissl, and Prussian blue staining for pathological changes, neuronal damage, and iron deposition in the brain tissue of mice in each group. Scale bar, 40 μm; B: Representative photomicrographs of Nissl staining of surviving neurons in the hippocampal region, and statistical analysis of Nissl bodies in each group; C: Representative photomicrographs of Prussian blue staining of surviving neurons in the hippocampal region, and statistical analysis of iron deposition in each group. aP < 0.05, compared with the sham group; bP < 0.05, compared with the amyloid β group; cP < 0.05, compared with the dexmedetomidine group. Aβ: Amyloid β; Dex: Dexmedetomidine; si-mTOR: Mammalian target of rapamycin siRNA; HE: Hematoxylin and eosin; HNs: Hippocampal neurons.
- Citation: Qiao L, Li G, Yuan HX. Dexmedetomidine mediates the mechanism of action of ferroptosis in mice with Alzheimer’s disease by regulating the mTOR-TFR1 pathway. World J Psychiatry 2023; 13(8): 511-523
- URL: https://www.wjgnet.com/2220-3206/full/v13/i8/511.htm
- DOI: https://dx.doi.org/10.5498/wjp.v13.i8.511