Copyright
©The Author(s) 2025.
World J Clin Pediatr. Mar 9, 2025; 14(1): 100938
Published online Mar 9, 2025. doi: 10.5409/wjcp.v14.i1.100938
Published online Mar 9, 2025. doi: 10.5409/wjcp.v14.i1.100938
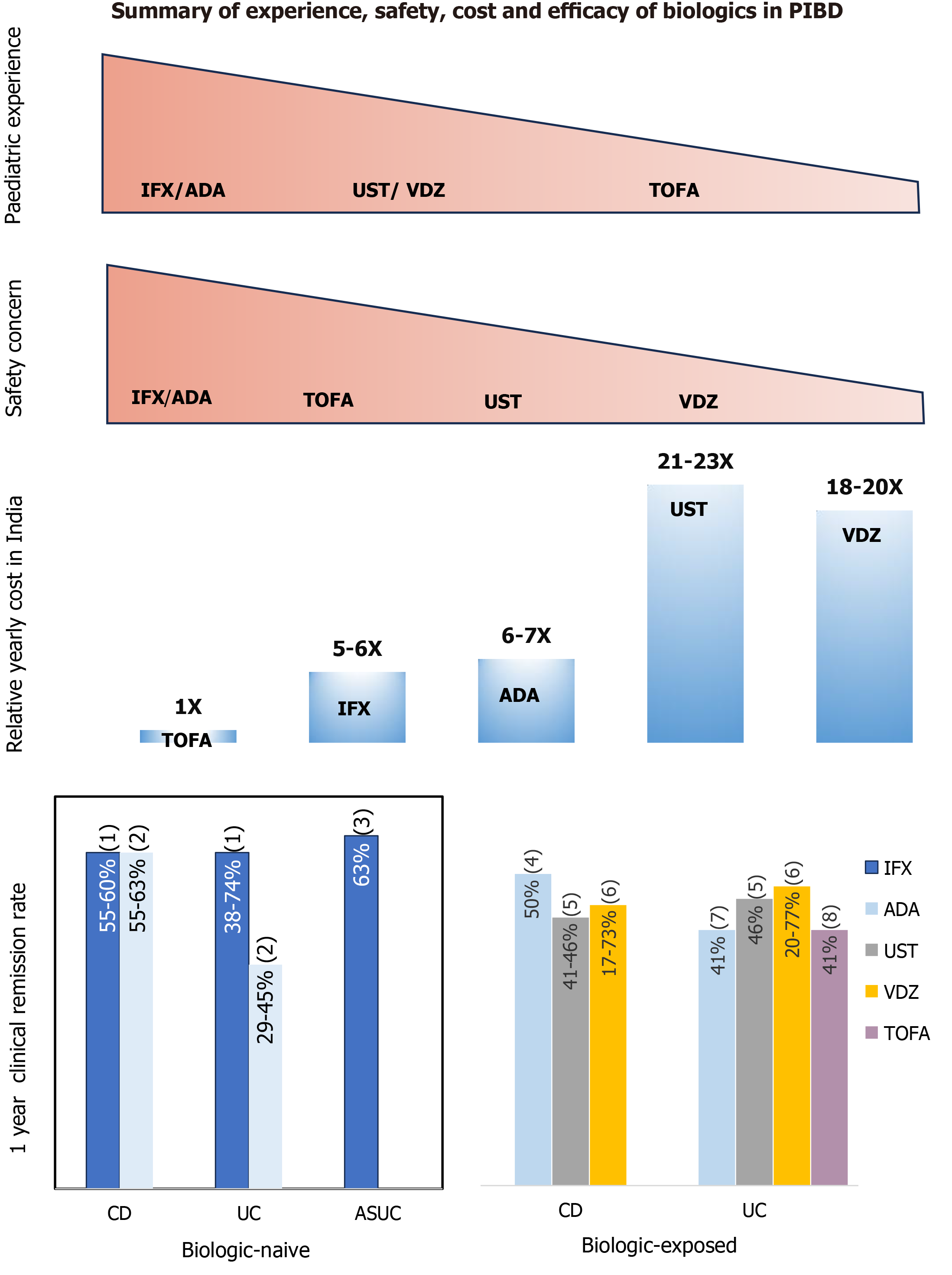
Figure 3 Comparative safety, relative annual cost (in India) and efficacy profile of various biologics in pediatric inflammatory bowel disease.
PIBD: Pediatric inflammatory bowel disease; IFX: Infliximab; ADA: Adalimumab; UST: Ustekinumab; VDZ: Vedolizumab; TOFA: Tofacitinib; CD: Crohn’s disease; UC: Ulcerative colitis; ASUC: Acute severe ulcerative colitis; (1): Response rates to biologics taken from reference[21,25,26,31,34,103]; (2): Response rates to biologics taken from reference[30,31,68]; (3): Response rates to biologics taken from reference[62]; (4): Response rates to biologics taken from reference[36]; (5): Response rates to biologics taken from reference[53,56]; (6): Response rates to biologics taken from reference[51]; (7): Response rates to biologics taken from reference[33]; (8): Response rates to biologics taken from reference[57].
- Citation: Samanta A, Srivastava A. Biologics in the management of pediatric inflammatory bowel disease: When and what to choose. World J Clin Pediatr 2025; 14(1): 100938
- URL: https://www.wjgnet.com/2219-2808/full/v14/i1/100938.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v14.i1.100938