Copyright
©2012 Baishideng Publishing Group Co.
World J Clin Oncol. Jun 10, 2012; 3(6): 82-91
Published online Jun 10, 2012. doi: 10.5306/wjco.v3.i6.82
Published online Jun 10, 2012. doi: 10.5306/wjco.v3.i6.82
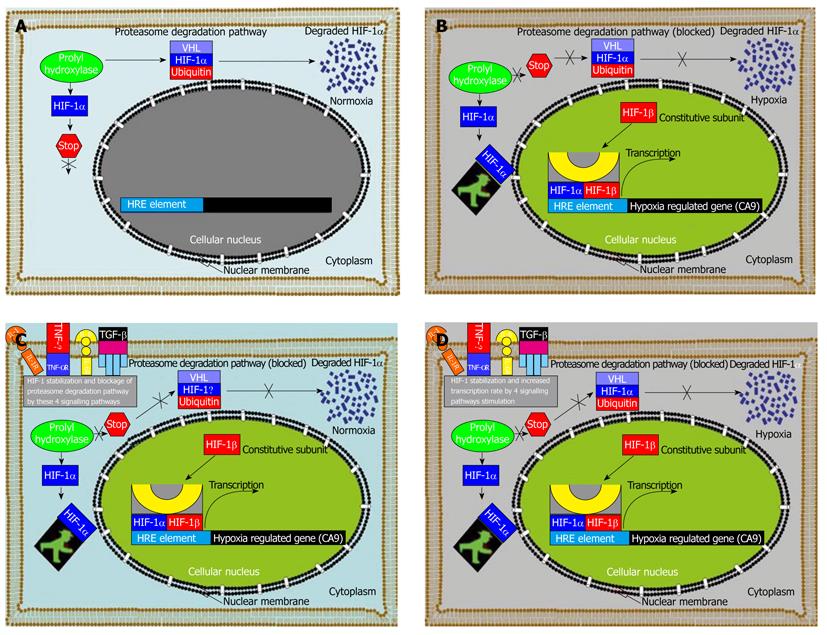
Figure 6 Hypoxia-inducible factor-1 alpha induced regulation of hypoxia-induced carbonic anhydrase 9 expression in human tumor cells without or with stimulation.
A: Under normoxic conditions in the tumour cell microenvironment, hypoxia-inducible factor-1 alpha (HIF-1α) is rapidly degraded via the von Hippel-Lindau tumour suppressor gene product (pVHL) - mediated proteasome pathway; B: Following a shift in tumour environment aeration conditions from normoxic to hypoxic aeration conditions, HIF-1α subunit becomes stable and translocates into the cellular nucleus and interacts with co-activators of which its transcription machinery consists if e.g. p300/CBP to modulate the transcriptional activity of numerous hypoxia inducible genes, such as carbonic anhydrase 9 (CA9) in our case, and about 61 other hypoxia induced genes[51]; C: When the cells are stimulated under normoxia with either interleukin (IL)-1, IL-6, tumor necrosis factor-alpha (TNF-α) or transforming growth factor-beta (TGF-β), the transcription factor HIF-1α subunit becomes stable despite the oxygenation status of the tumour environment and translocates into the cellular nucleus and interacts with co-activators of which its transcription machinery consists of e.g. p300/CBP to modulate the transcriptional activity of CA9 with a similar expression to that under hypoxia; D: Experimental stimulation with either IL-1, IL-6, TNF-α or TGF-β1 under hypoxia increases the CA9 level to almost double the expression rate under hypoxic conditions with the stimulation of these cytokines due to increased HIF-1α translocation into the nucleus and increased binding rate to the hypoxia response element element within the CA9 promoter region.
-
Citation: Kockar F, Yildrim H, Sagkan RI, Hagemann C, Soysal Y, Anacker J, Hamza AA, Vordermark D, Flentje M, Said HM. Hypoxia and cytokines regulate carbonic anhydrase 9 expression in hepatocellular carcinoma cells
in vitro . World J Clin Oncol 2012; 3(6): 82-91 - URL: https://www.wjgnet.com/2218-4333/full/v3/i6/82.htm
- DOI: https://dx.doi.org/10.5306/wjco.v3.i6.82