Copyright
©2011 Baishideng Publishing Group Co.
World J Cardiol. Sep 26, 2011; 3(9): 281-302
Published online Sep 26, 2011. doi: 10.4330/wjc.v3.i9.281
Published online Sep 26, 2011. doi: 10.4330/wjc.v3.i9.281
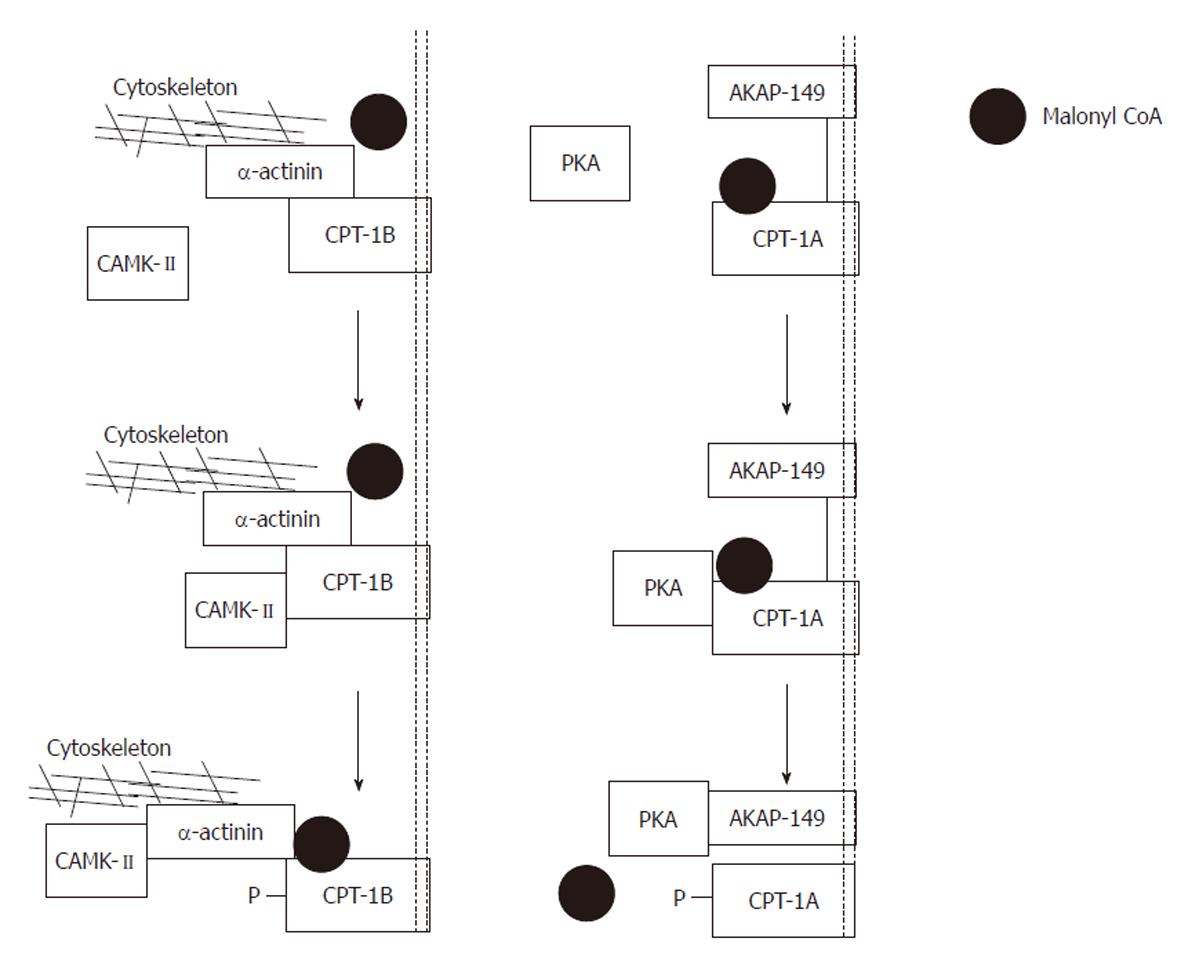
Figure 10 Proposed model of the actions of protein kinase A and calmodulin-dependent protein kinase-II.
Left panel: Exogenously applied protein kinase A (PKA) phosphorylates carnitine palmitoyltransferase (CPT)-1A and is then captured by its scaffolding protein. Phosphorylation produces a conformational change tightening the interaction between A-kinase anchoring protein (AKAP)-149 and CPT-1A. As a result, malonyl CoA is denied access to its binding site, and the sensitivity of CPT-1 to malonyl CoA is reduced. Note that CPT-1 and AKAP-149 are both anchored in the mitochondrial membrane; Right panel: Exogenously applied calmodulin-dependent protein kinase (CAMK)-II phosphorylates CPT-1B and is then captured by its scaffolding protein α-actinin. Phosphorylation produces a conformational change, loosening the interaction between α-actinin and CPT-1B. As a result, malonyl CoA has improved access to its binding site and the sensitivity of CPT-1 to malonyl CoA is increased. Note that CPT-1B is anchored to the mitochondrial membrane whereas α-actinin is anchored to the cytoskeleton. Modified from: Figure 4, supplementary information, Sharma et al[108].
- Citation: Sharma V, McNeill JH. Parallel effects of β-adrenoceptor blockade on cardiac function and fatty acid oxidation in the diabetic heart: Confronting the maze. World J Cardiol 2011; 3(9): 281-302
- URL: https://www.wjgnet.com/1949-8462/full/v3/i9/281.htm
- DOI: https://dx.doi.org/10.4330/wjc.v3.i9.281