Copyright
©The Author(s) 2015.
World J Biol Chem. Nov 26, 2015; 6(4): 366-378
Published online Nov 26, 2015. doi: 10.4331/wjbc.v6.i4.366
Published online Nov 26, 2015. doi: 10.4331/wjbc.v6.i4.366
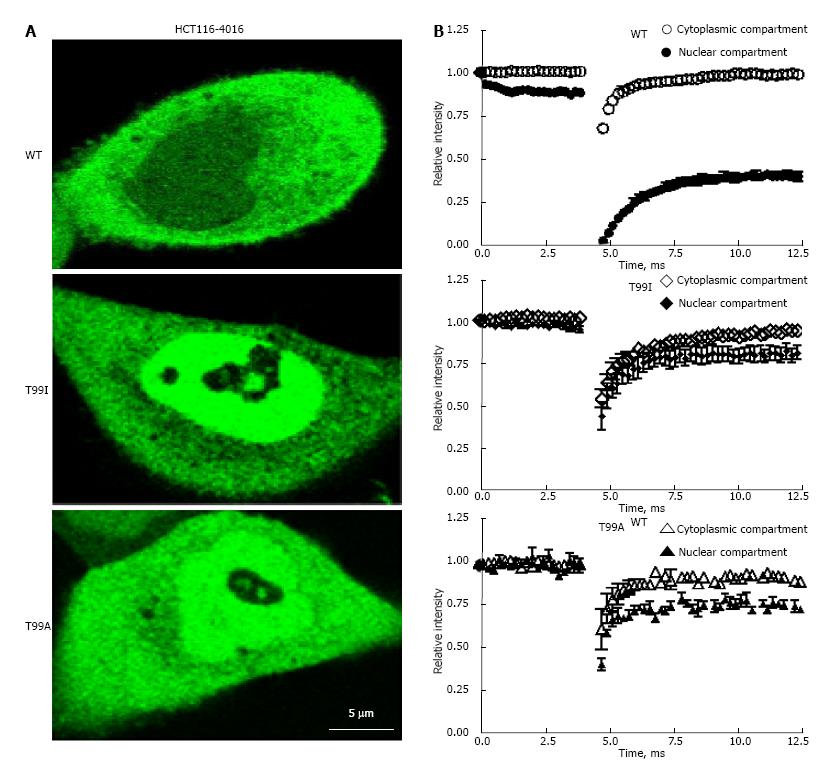
Figure 6 Intranuclear mobility of wild type and mutated variants of enhanced green fluorescent protein-glyceraldehyde 3-phosphate dehydrogenase in HCT116-4016 cells after genotoxic stress.
A: FRAP analysis of fluorescent fusion protein EGFP-GAPDH in subcellular compartments of HCT116-4016 cells. Confocal images were collected from transfected cells expressing WT-EGFP-GAPDH (WT), EGFP-GAPDH-T99I (T99I), and EGFP-GAPDH-T99A (T99A) after treatment with 1 μmol/L araC for 24 h; B: Recovery rate of wild type and mutant EGFP-GAPDH fusion proteins after photo bleaching. Open symbols, cytoplasmic compartment, and closed symbols, nuclear compartment. The dynamic parameters mobile fraction (1-Mf), half-time of equilibration t(1/2) (s), and diffusion coefficients D were calculated from FRAP experiments, as described in “Materials and Methods” and summarized in Table 3. Where not seen, the error bars are smaller than the data point symbols. EGFP: Enhanced green fluorescent protein; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase; GFP: Green fluorescent protein; FRAP: Fluorescence recovery after photobleaching.
- Citation: Phadke M, Krynetskaia N, Mishra A, Barrero C, Merali S, Gothe SA, Krynetskiy E. Disruption of NAD+ binding site in glyceraldehyde 3-phosphate dehydrogenase affects its intranuclear interactions. World J Biol Chem 2015; 6(4): 366-378
- URL: https://www.wjgnet.com/1949-8454/full/v6/i4/366.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v6.i4.366