Copyright
©2014 Baishideng Publishing Group Inc.
World J Biol Chem. May 26, 2014; 5(2): 240-253
Published online May 26, 2014. doi: 10.4331/wjbc.v5.i2.240
Published online May 26, 2014. doi: 10.4331/wjbc.v5.i2.240
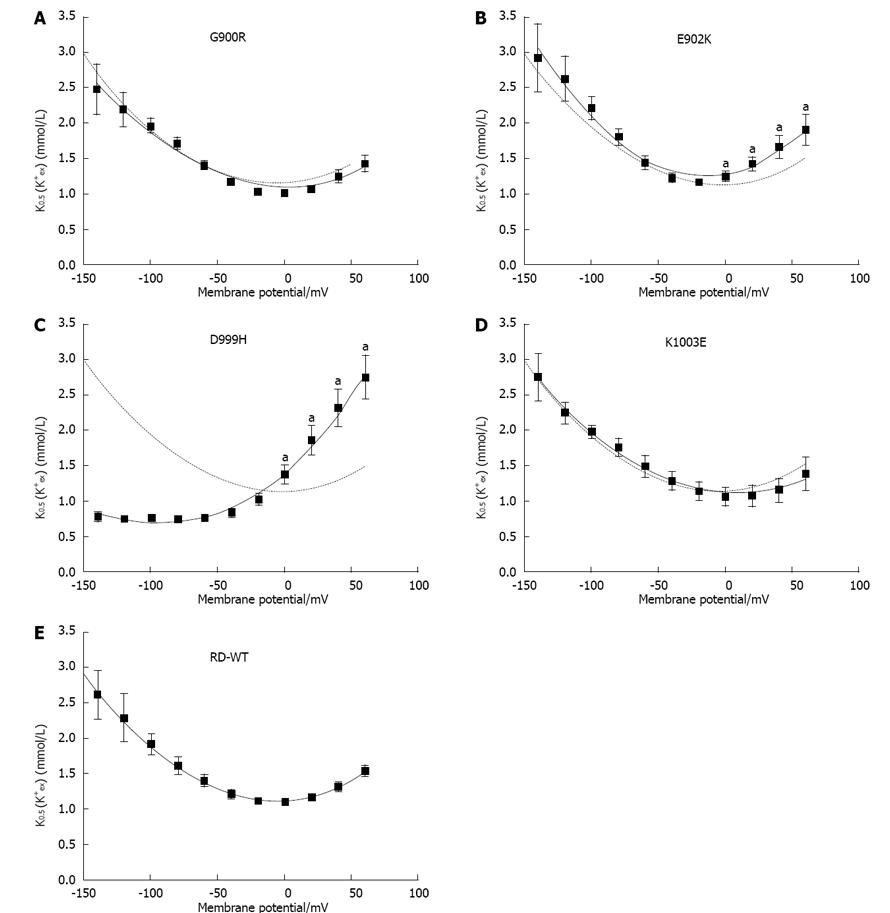
Figure 3 Apparent K+ affinity.
A-E: K0.5 values for the [K+]ex dependence of stationary currents at different membrane potentials for the RD-WT enzyme (E) and the mutants G900R (A), E902K (B), D999H (C) and K1003E (D), as calculated from fits of a Hill function to the data in Figure 2, respectively. Data were approximated with polynomial functions of second or third (D999H) grade to determine the minimum. The curve derived from RD-WT data is superimposed as dotted line for comparison. An “a” indicates that the data point was significantly different from the RD-WT data (aP < 0.05 vs RD-WT, Student’s t-test). Data are means ± SE obtained from 5-15 cells of at least three batches.
- Citation: Spiller S, Friedrich T. Functional analysis of human Na+/K+-ATPase familial or sporadic hemiplegic migraine mutations expressed in Xenopus oocytes. World J Biol Chem 2014; 5(2): 240-253
- URL: https://www.wjgnet.com/1949-8454/full/v5/i2/240.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v5.i2.240