Copyright
©2014 Baishideng Publishing Group Inc.
World J Biol Chem. May 26, 2014; 5(2): 141-160
Published online May 26, 2014. doi: 10.4331/wjbc.v5.i2.141
Published online May 26, 2014. doi: 10.4331/wjbc.v5.i2.141
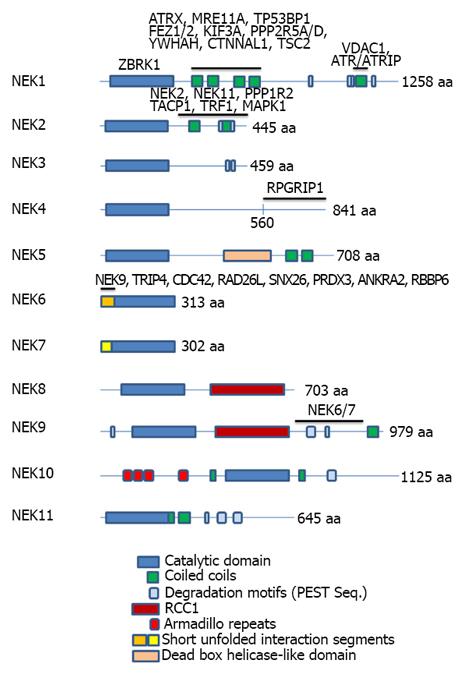
Figure 1 Representation of the domain organization of the eleven human Neks depicting the domain regions for selected protein interactions.
The gene symbols corresponding to interacting proteins are shown above the Neks primary structure regions with which they have been found to interact. The list of interactors is not intended to be complete but is necessarily shorter than the list of all proteins known in the literature to interact with Neks (e.g., see Figure 2), since, for the majority of interactors, the location of interaction in the Neks has not been reported. Different repeated domains have been indicated by the color code at the bottom of the figure. The lengths of the full proteins are indicated by number of amino acids (aa) at the C-terminal of the proteins. At least two isoforms of Nek1, 2, 3 and three of Nek4 and 11, all generated by alternative splicing, have been reported and known functional distinctions have been briefly discussed in the text, where feasible. References for the proteins and their mapped interactors: Nek1[2,13,25]; Nek2[116,121-124]; Nek4[53]; Nek6[3]; Nek9[66]. Nek: Never in mitosis-gene A-related kinases.
- Citation: Meirelles GV, Perez AM, de Souza EE, Basei FL, Papa PF, Melo Hanchuk TD, Cardoso VB, Kobarg J. “Stop Ne(c)king around”: How interactomics contributes to functionally characterize Nek family kinases. World J Biol Chem 2014; 5(2): 141-160
- URL: https://www.wjgnet.com/1949-8454/full/v5/i2/141.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v5.i2.141