Copyright
©2014 Baishideng Publishing Group Co.
World J Biol Chem. Feb 26, 2014; 5(1): 26-39
Published online Feb 26, 2014. doi: 10.4331/wjbc.v5.i1.26
Published online Feb 26, 2014. doi: 10.4331/wjbc.v5.i1.26
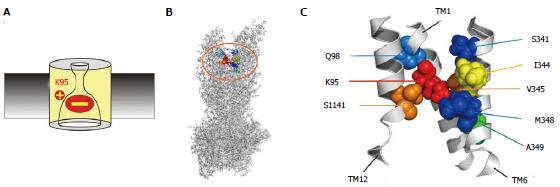
Figure 3 Location of amino acid residues key for blocker interactions in the pore inner vestibule.
A: The positively charged side chain of lysine residue K95 is essential for block, due to electrostatic attraction between this positive charge and the negatively charged blocker. However, this important charge can also be supported by other amino acid side chains that line the pore inner vestibule. B, C: Sites that have been shown to host positive charge that can support block are shown in an atomic homology model of the whole cystic fibrosis transmembrane conductance regulator protein (B) and in a detailed view of the central portions of TMs 1, 6 and 12 (C) the area highlighted in (B). The endogenous positively charged side chain of K95 is shown in red; those residues that were deemed best able to support this functionally important positive charge in orange (V345, S1141) or yellow (I344); and those that were able to host this positive charge to a lesser extent in blue (Q98, S341, M348) or green (A349). The homology model used here is the “channel like” conformation presented by ref[20] and shown in Figure 1A; other models give similar relative positions of these pore-lining side chains.
- Citation: Linsdell P. Cystic fibrosis transmembrane conductance regulator chloride channel blockers: Pharmacological, biophysical and physiological relevance. World J Biol Chem 2014; 5(1): 26-39
- URL: https://www.wjgnet.com/1949-8454/full/v5/i1/26.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v5.i1.26