Copyright
©2010 Baishideng Publishing Group Co.
World J Biol Chem. Aug 26, 2010; 1(8): 239-247
Published online Aug 26, 2010. doi: 10.4331/wjbc.v1.i8.239
Published online Aug 26, 2010. doi: 10.4331/wjbc.v1.i8.239
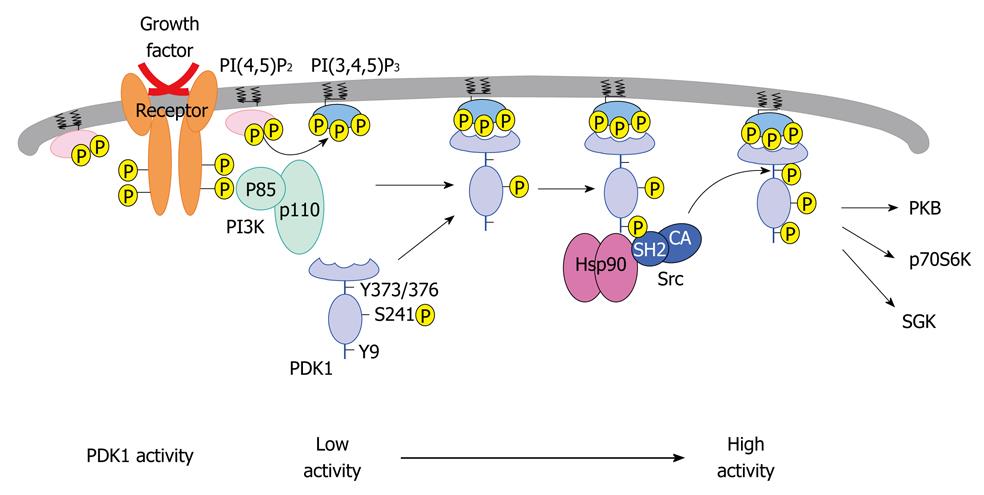
Figure 2 Proposed mechanism for the regulation of 3-phosphoinositide-dependent protein kinase-1 phosphorylation and stability.
3-phosphoinositide-dependent protein kinase-1 (PDK1) autophosphorylates itself on Ser-241. In the presence of pervanadate or insulin, PDK1 is phosphorylated on Tyr-9 and Tyr-373/376 with the help of Src and heat shock protein 90 (Hsp90). Tyrosine phosphorylation further increases PDK1 catalytic activity. In the absence of Hsp90 interaction, PDK1 is promoted towards proteasome-dependent degradation. This figure was adopted from Yang et al[41], 2008. PI3K: Phosphoinositide 3-kinase; PI(4,5)P2: Phosphatidylinositol 3,4 bisphosphate; PI(3,4,5)P3: Phosphatidylinositol 3,4,5 trisphosphate; CA: Constitutively active; PH: Pleckstrin homology; PKB: Protein kinase B; SGK: Serum and glucocorticoid-inducible kinase.
- Citation: Li Y, Yang KJ, Park J. Multiple implications of 3-phosphoinositide-dependent protein kinase 1 in human cancer. World J Biol Chem 2010; 1(8): 239-247
- URL: https://www.wjgnet.com/1949-8454/full/v1/i8/239.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v1.i8.239