Copyright
©2014 Baishideng Publishing Group Inc.
World J Diabetes. Oct 15, 2014; 5(5): 606-629
Published online Oct 15, 2014. doi: 10.4239/wjd.v5.i5.606
Published online Oct 15, 2014. doi: 10.4239/wjd.v5.i5.606
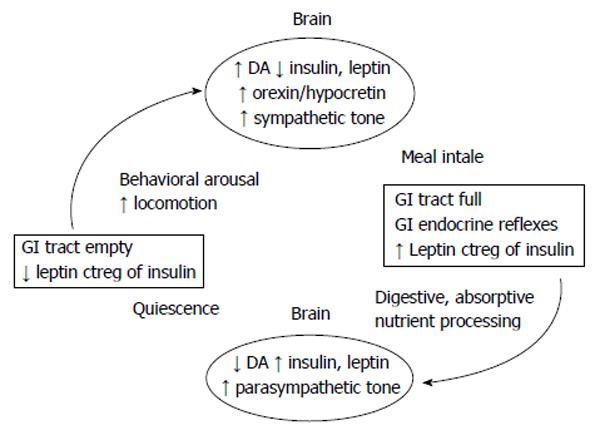
Figure 6 The conceptual model of the linkage between nonhomeostatic meal eating and nonhomeostatic facilitation of physical activity.
Completion of gastrointestinal (GI) transit of food removes the inhibitory influence of volumetric and nutritional afferent information mediated by the vagus nerve from reaching nucleus tractus solitaries and DA and opioidergic brain centers of reward.This allows activation of sympathetic actions over fuel mobilization, full operation of behavioral arousal, nonhomeostatic increase in locomotion in quest of food associated with activation of orexin/hypocretin and ghrelin. Completion of nonhomeostatically controled meal results in filling of the stomach, activation of GI nutrient sensing, and increases in postprandial plasma concentrations of insulin and leptin. Vagal projections of this information to the brain reinstate the inhibition over autonomic sympathetic actions and activate parasympathetic control of food digestion and absorption and behavioral quiescence. It is probable that weight loss increases postprandial events linked by the left arrow, and that obesity increases the postprandial events linked by right arrow. Additional consequences of weight loss and weight gain are mediated by changes in tissue sensitivities to leptin and insulin actions altering the prevailing sympathovagal balance and illustrated in Figure 5. DA: Dopaminergic.
- Citation: Borer KT. Counterregulation of insulin by leptin as key component of autonomic regulation of body weight. World J Diabetes 2014; 5(5): 606-629
- URL: https://www.wjgnet.com/1948-9358/full/v5/i5/606.htm
- DOI: https://dx.doi.org/10.4239/wjd.v5.i5.606