Copyright
©2011 Baishideng Publishing Group Co.
World J Diabetes. Nov 15, 2011; 2(11): 176-188
Published online Nov 15, 2011. doi: 10.4239/wjd.v2.i11.176
Published online Nov 15, 2011. doi: 10.4239/wjd.v2.i11.176
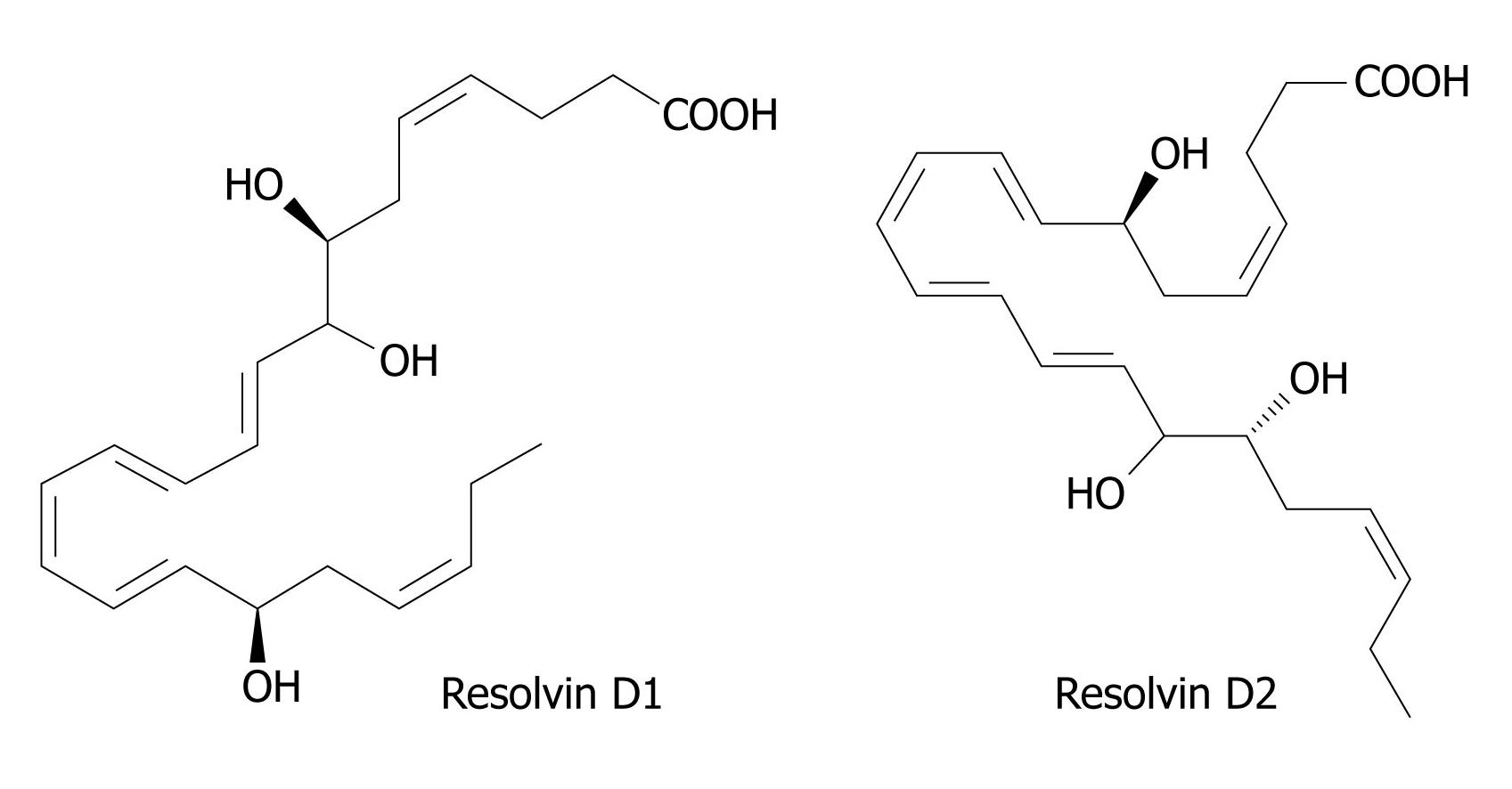
Figure 4 Structures of Resolvin D1 and D2.
DHA is converted to 17R-resolvins by a similar aspirin-triggered mechanism similar to the scheme shown in Figure 2. In the absence of aspirin, COX-2 of endothelial cells converts DHA to 13S-hydroxy-DHA. In the presence of aspirin, the initial product is 17R-hydroxy-DHA, which is converted to 7S-hydroperoxy, 17R-hydroxy-DHA by the action of a lipoxygenase, and thence via an epoxy intermediate to epimeric resolvins D1 and D2. An alternative lipoxygenase-generated intermediate, 4S-hydroperoxy, 17R-hydroxy-DHA, is transformed via an epoxide to epimeric resolvins D3 and D4. 17S Resolvins of the D series are produced in cells in the absence of aspirin by a reaction catalyzed in the first step by a lipoxygenase. COX: Cyclo-oxygenase; DHA: Docosahexaenoic acid.
- Citation: Das UN. A defect in the activities of Δ6 and Δ5 desaturases and pro-resolution bioactive lipids in the pathobiology of non-alcoholic fatty liver disease. World J Diabetes 2011; 2(11): 176-188
- URL: https://www.wjgnet.com/1948-9358/full/v2/i11/176.htm
- DOI: https://dx.doi.org/10.4239/wjd.v2.i11.176