Copyright
©The Author(s) 2023.
World J Stem Cells. May 26, 2023; 15(5): 438-452
Published online May 26, 2023. doi: 10.4252/wjsc.v15.i5.438
Published online May 26, 2023. doi: 10.4252/wjsc.v15.i5.438
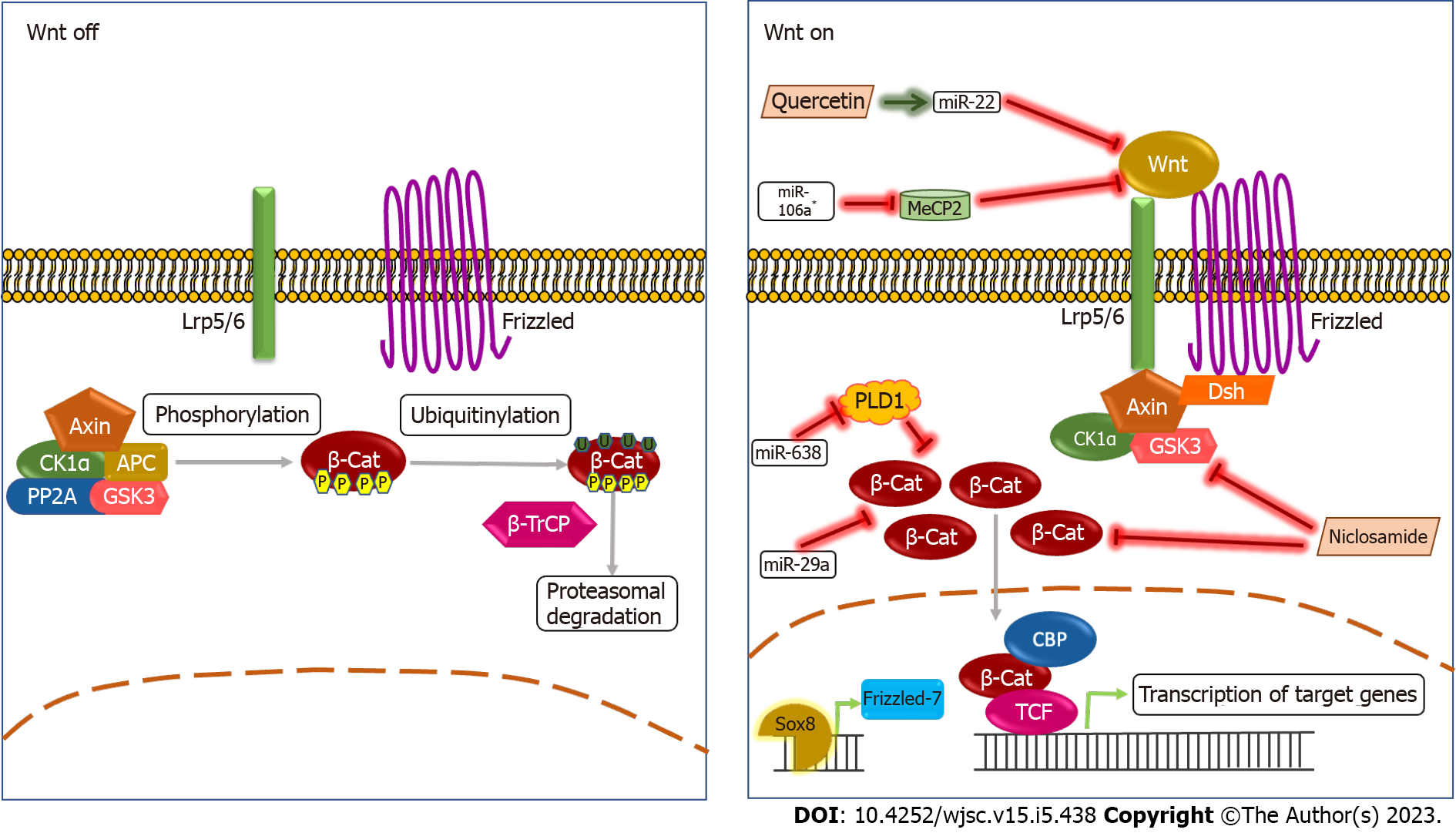
Figure 1 The Wnt signaling pathway and cancer stemness.
In the absence of a Wnt ligand, in the canonical pathway,, a complex of Axin, CK1α, APC, PP2A, and GSK3 (termed destruction complex) phosphorylates β-catenin targeting it for ubiquitinylation, that leads to its proteasomal degradation. When the Wnt ligand binds to the Frizzled receptor and LRP5/6 co-receptor, the destruction complex gets localized to the receptor, preventing the degradation of β-catenin that localizes to the nucleus that further activates transcription of the target genes. It has been reported that miRNAs such as miR-29a, miR-638, and miR-106a* reduce levels of β-catenin and Wnt ligand. The miR-638 targets PLD1, a generally accepted oncogene, leading to the reduction β-catenin levels[49]. The miR-29a also directly causes a reduction in β-catenin levels in TSCC cell lines[48]. The miRNA miR-106a* causes a reduction in MeCP2 (a gene expression regulator and oncogene) levels that inhibit the binding of Wnt ligand to the receptor that in turn causes downregulation of the Wnt pathway[50]. Chemical compounds such as Quercetin and Niclosamide downregulate the Wnt signaling pathway. Quercetin causes an increase in levels of miR-22 that in turn inhibits the Wnt1/β-catenin axis[51], while Niclosamide directly binds to DVL2, phosphorylated GSK3β, and Cyclin D1 that reduces levels of β-catenin[52]. Sox8 is shown to activate the Wnt pathway by inducing the expression of Frizzled-7[53].
- Citation: Joshi P, Waghmare S. Molecular signaling in cancer stem cells of tongue squamous cell carcinoma: Therapeutic implications and challenges. World J Stem Cells 2023; 15(5): 438-452
- URL: https://www.wjgnet.com/1948-0210/full/v15/i5/438.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i5.438