Copyright
©The Author(s) 2023.
World J Gastroenterol. May 14, 2023; 29(18): 2784-2797
Published online May 14, 2023. doi: 10.3748/wjg.v29.i18.2784
Published online May 14, 2023. doi: 10.3748/wjg.v29.i18.2784
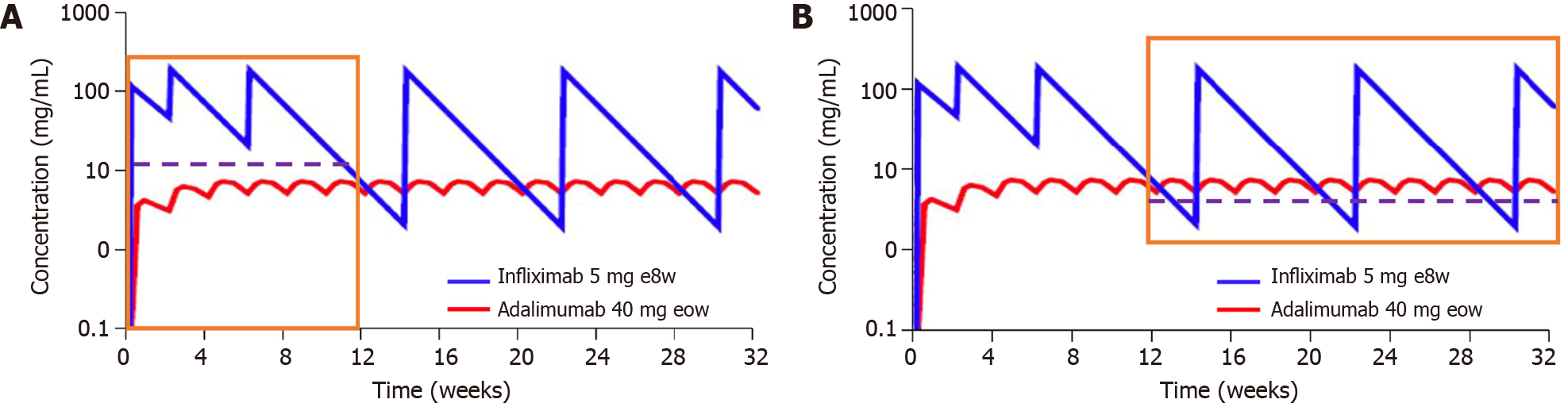
Figure 1 The pharmacokinetic profile of an intravenously or subcutaneously administered anti-tumor necrosis factor agent.
A: According to a theoretical induction dosing regimen; B: According to a theoretical maintenance dosing regimen. TNF: tumor necrosis factor. Citation: Gibson DJ, Ward MG, Rentsch C, Friedman AB, Taylor KM, Sparrow MP, Gibson PR. Review article: determination of the therapeutic range for therapeutic drug monitoring of adalimumab and infliximab in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2020; 51: 612-628. Copyright ©John Wiley & Sons Ltd. 2020. Published by John Wiley & Sons[31].
- Citation: Kim ES, Kang B. Infliximab vs adalimumab: Points to consider when selecting anti-tumor necrosis factor agents in pediatric patients with Crohn’s disease. World J Gastroenterol 2023; 29(18): 2784-2797
- URL: https://www.wjgnet.com/1007-9327/full/v29/i18/2784.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i18.2784