Copyright
©The Author(s) 2017.
World J Gastroenterol. Mar 21, 2017; 23(11): 1974-1979
Published online Mar 21, 2017. doi: 10.3748/wjg.v23.i11.1974
Published online Mar 21, 2017. doi: 10.3748/wjg.v23.i11.1974
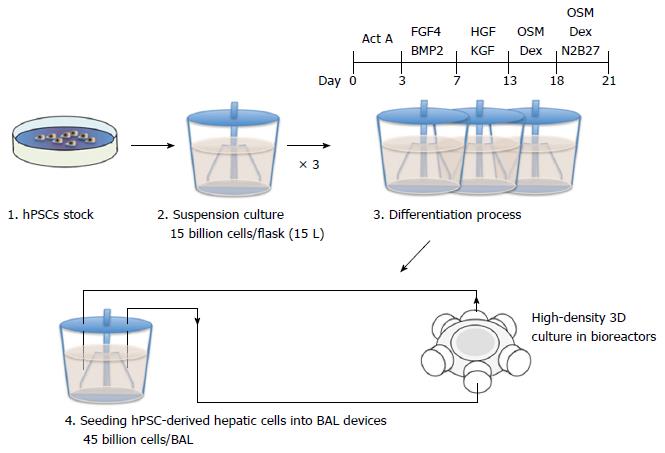
Figure 3 A strategy and cell number estimate of human pluripotent stem cells-derived hepatic cells in the mass production of bioartificial liver devices.
Undifferentiated human pluripotent stem cells (hPSCs) can be expanded in a 15 L suspension culture system up to a maximum of 15 billion cells[37]. Three of these suspension culture flasks will be required to prepare 45 billion cells for a clinical-scale bioartificial liver (BAL) device. After inducing hepatic differentiation, the hPSC-derived hepatic cells will be cultured at high density in bioreactors to generate a BAL device.
- Citation: Sakiyama R, Blau BJ, Miki T. Clinical translation of bioartificial liver support systems with human pluripotent stem cell-derived hepatic cells. World J Gastroenterol 2017; 23(11): 1974-1979
- URL: https://www.wjgnet.com/1007-9327/full/v23/i11/1974.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i11.1974