Copyright
©The Author(s) 2016.
World J Gastroenterol. Dec 7, 2016; 22(45): 9921-9932
Published online Dec 7, 2016. doi: 10.3748/wjg.v22.i45.9921
Published online Dec 7, 2016. doi: 10.3748/wjg.v22.i45.9921
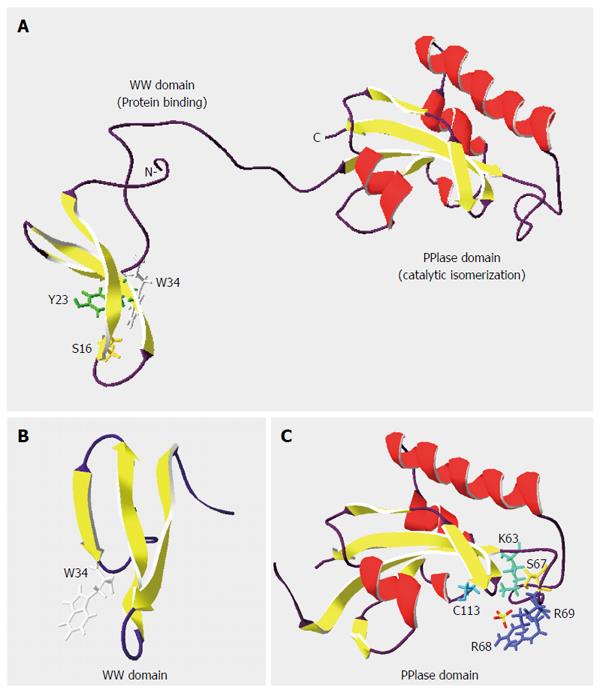
Figure 1 Structure of peptidyl-prolyl-isomerase PIN1 protein.
Ribbon diagrams of (A) PIN1 (NCBI Structure No. 1NMV), (B) WW binding domain (NCBI Structure No. 1I8H), and (C) PPIase catalytic domain (NCBI Structure No. 1NMW) were drawn with the Swiss-Pdb Viewer[11,15,115,116]. α-helices and β-strands are denoted by coils and arrows, respectively. Residues Ser(S)16, Tyr(Y)23 and Trp(W)34 in the WW domain are critical for phospho-protein binding, while residues Lys(K)63, Ser(S)67, Arg(R)68/69 and Cys(C)113 contribute to the PPIase activity. Adapted from thesis: Identification and characterization of PIN1 binding partners, HKU 2010.
- Citation: Cheng CW, Leong KW, Tse E. Understanding the role of PIN1 in hepatocellular carcinoma. World J Gastroenterol 2016; 22(45): 9921-9932
- URL: https://www.wjgnet.com/1007-9327/full/v22/i45/9921.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i45.9921