Copyright
©2014 Baishideng Publishing Group Inc.
World J Gastroenterol. Aug 7, 2014; 20(29): 9775-9827
Published online Aug 7, 2014. doi: 10.3748/wjg.v20.i29.9775
Published online Aug 7, 2014. doi: 10.3748/wjg.v20.i29.9775
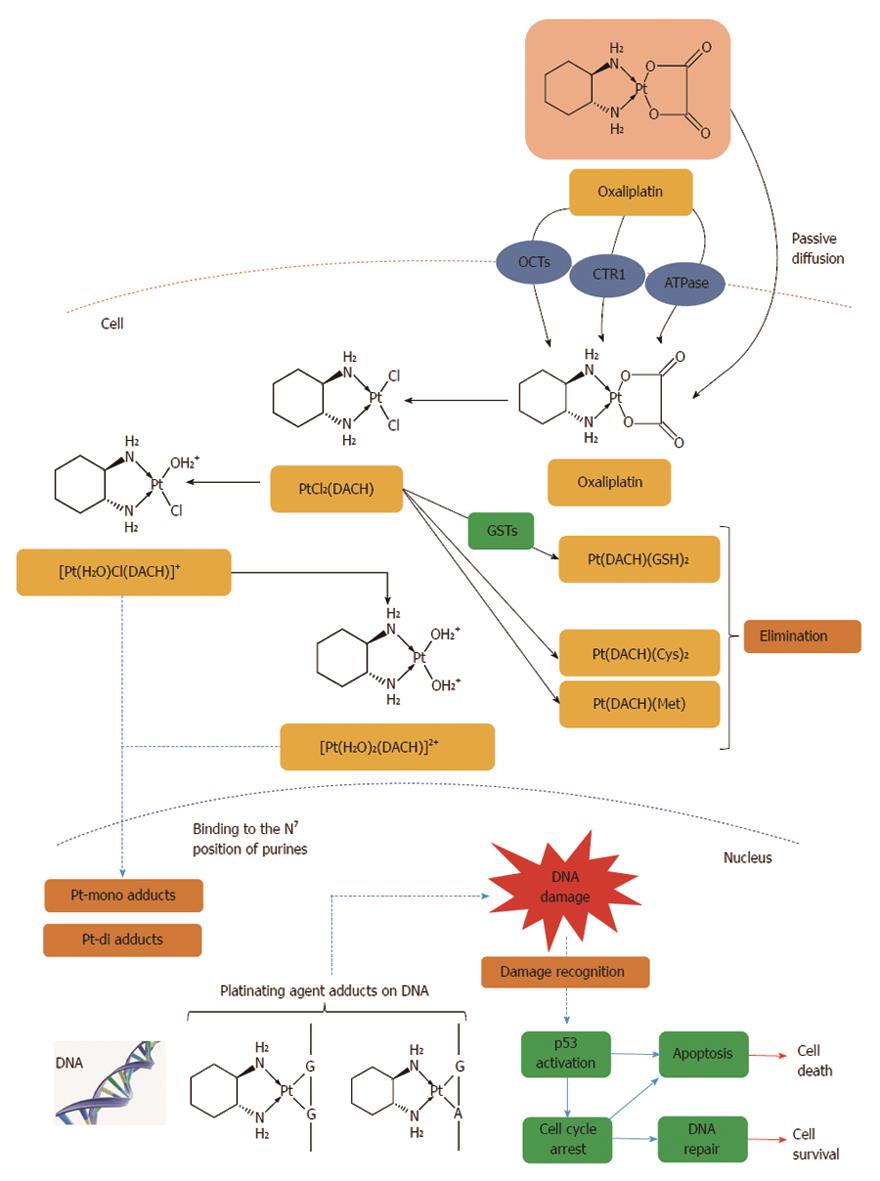
Figure 8 Intracellular drug accumulation.
The free fraction of oxaliplatin is biotransformed non-enzymatically and subsequently forms complexes with chloride, glutathione (GSH), methionine (Met) and cysteine (Cys). Oxaliplatin undergoes non-enzymatic conversion in physiologic solutions to active derivatives via displacement of the labile oxalate ligand. Several transient reactive species are formed, including monoaquo DACH (1,2-diaminocyclohexane) platinum [Pt(H2O)Cl(DACH)]+ and diaquo DACH platinum [Pt(H2O)2(DACH)]2+, which covalently bind with macromolecules. There is no evidence of cytochrome P450-mediated metabolism in vitro. The major route of platinum elimination is renal excretion. The main mechanism of action is mediated through the formation of DNA adducts which is thought to be related to the anti-tumour effects of oxaliplatin. An important factor is the induction of apoptosis by the primary DNA-Pt lesions, which is possibly enhanced by the contribution of targets other than DNA. Several influx and efflux transporters such as organic cation transporters (OCTs) 1, 2 and 3 (SLC22A1, SLC22A2 and SLC22A3), copper efflux transporters (CTRs), P-type ATPases, ATP7A and ATP7B have been identified, which may play an important role in determining tumour sensitivity and/or resistance to oxaliplatin[408].
- Citation: Panczyk M. Pharmacogenetics research on chemotherapy resistance in colorectal cancer over the last 20 years. World J Gastroenterol 2014; 20(29): 9775-9827
- URL: https://www.wjgnet.com/1007-9327/full/v20/i29/9775.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i29.9775