Copyright
©2014 Baishideng Publishing Group Inc.
World J Gastroenterol. Aug 7, 2014; 20(29): 9775-9827
Published online Aug 7, 2014. doi: 10.3748/wjg.v20.i29.9775
Published online Aug 7, 2014. doi: 10.3748/wjg.v20.i29.9775
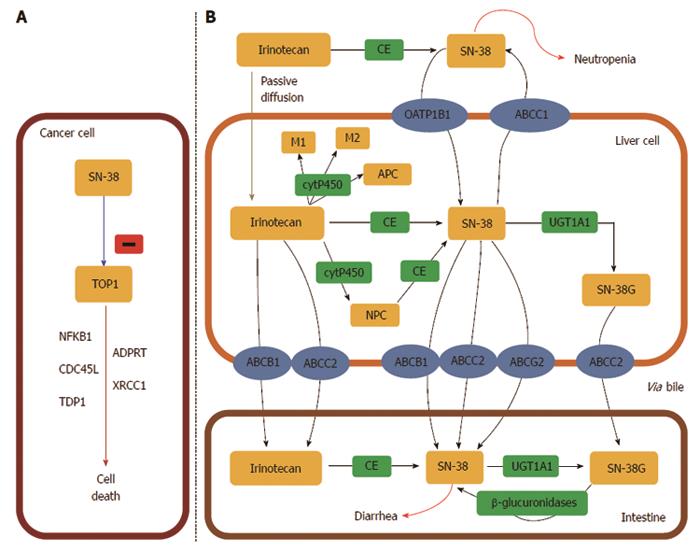
Figure 7 UDP-glycosyltransferase 1 family.
A: The active metabolite of irinotecan, SN-38, is a DNA topoisomerase I (TOP1) inhibitor which leads to cancer cell death. TOP1 is a nuclear enzyme required in replication, responsible for unwinding DNA and preventing lethal strand breaks. SN-38 is cytotoxic and destabilizes the TOP1-DNA covalent complex formed in colorectal cancer cells. SN-38 causes irreversible double strand breaks which lead to S phase arrest followed by cell death. To do so, SN-38 attaches to the complexes and blocks future replication forks preventing repair of double strand breaks[405]; B: Irinotecan uptake and transport into the liver is facilitated by: OATP1B1 (SLCO1B1), ABCB1, MRP1 (ABCC1), MRP2 (ABCC2), and MXR (ABCG2). Specifically, ABCB1 is present on the bile membrane and is responsible for the secretion of irinotecan and its metabolites into the liver[406]. Irinotecan is metabolized in the liver and converted to SN-38, the active metabolite and TOP1 inhibitor, by carboxylesterases (CE) mediated hydrolysis. SN-38 is then glucoronized to SN-38 glucuronic acid (SN-38G) and detoxified in the liver via conjugation by the UGT1A family, which releases SN-38G into the intestines for elimination[407]. Approximately 70% of SN-38 becomes SN-38G, which has 1/100 of the antitumour activity and is virtually inactive. In the intestinal lumen, bacterial β-glucuronidases can reverse the reaction and transform inactive SN-38G back into the active form SN-38. This factor contributes to varied toxicity, specifically dose limiting diarrhoea[198].
- Citation: Panczyk M. Pharmacogenetics research on chemotherapy resistance in colorectal cancer over the last 20 years. World J Gastroenterol 2014; 20(29): 9775-9827
- URL: https://www.wjgnet.com/1007-9327/full/v20/i29/9775.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i29.9775