Copyright
©2014 Baishideng Publishing Group Inc.
World J Gastroenterol. Aug 7, 2014; 20(29): 9775-9827
Published online Aug 7, 2014. doi: 10.3748/wjg.v20.i29.9775
Published online Aug 7, 2014. doi: 10.3748/wjg.v20.i29.9775
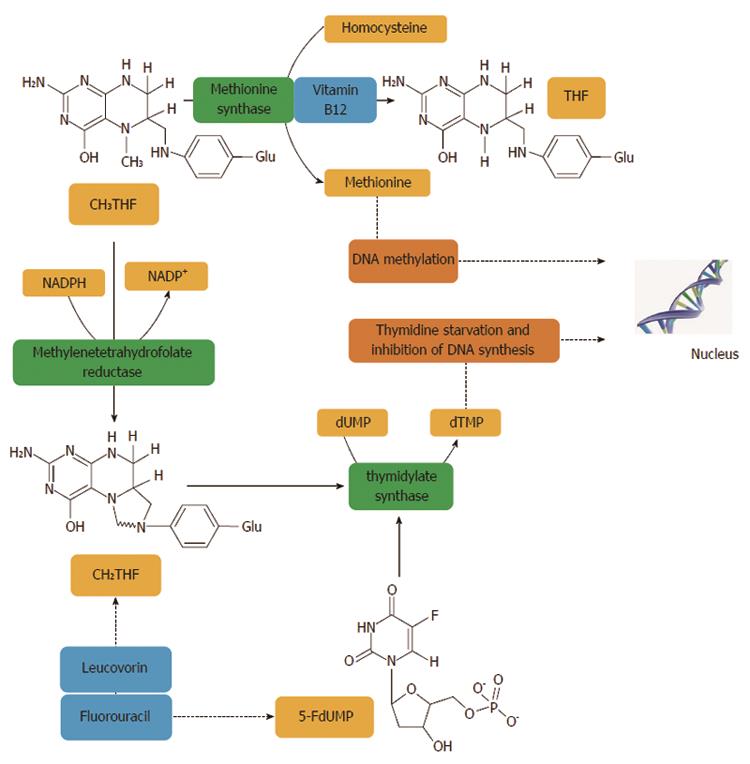
Figure 2 Methylentetrahydrofolate reductase plays an important role in the action of 5-fluorouracil, an inhibitor of thymidylate synthase.
Methylentetrahydrofolate reductase (MTHFR) catalyses a unidirectional reaction that lowers the levels of 5,10-methylenetetrahydrofolate (CH2THF) by increasing levels of 5-methyltetrahydrofolate (CH3THF) which is used for biological methylation. Other factors, such as vitamin B12 and homocysteine, are involved in biological methylation processes. The addition of folinic acid (leucovorin) to 5-FU improves the response rates and survival of CRC patients. Thymidylate synthase (TS) catalyses the reductive methylation of deoxyuridine monophosphate (dUMP) to deoxythymidine monophosphate (dTMP) with the reduced folate, CH2THF, as the methyl donor. This reaction provides the sole de novo source of thymidylate, which is necessary for DNA replication and repair. TS contains a nucleotide-binding site and a binding site for CH2THF. The 5-FU metabolite, FdUMP, binds to the nucleotide-binding site of TS, forming a stable ternary complex with the enzyme and CH2THF which blocks binding of the normal substrate dUMP, thereby inhibiting dTMP synthesis. Inhibition of thymidylate synthesis causes disruption of nucleotide levels that results in DNA damage[402].
- Citation: Panczyk M. Pharmacogenetics research on chemotherapy resistance in colorectal cancer over the last 20 years. World J Gastroenterol 2014; 20(29): 9775-9827
- URL: https://www.wjgnet.com/1007-9327/full/v20/i29/9775.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i29.9775