Copyright
©2014 Baishideng Publishing Group Co.
World J Gastroenterol. Mar 21, 2014; 20(11): 2785-2800
Published online Mar 21, 2014. doi: 10.3748/wjg.v20.i11.2785
Published online Mar 21, 2014. doi: 10.3748/wjg.v20.i11.2785
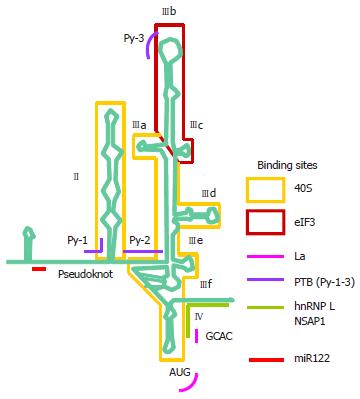
Figure 3 The hepatitis C virus internal ribosome entry site.
The hepatitis C virus (HCV) 5’ untranslated region (UTR) is divided into four stem-loop domains and sub-domains[61]. The internal ribosome entry site (IRES) element is made up of approximately 340 nucleotides and spans domain II and extends into the core-coding region encompassing a double pseudoknot fold[13,62-64]. The stem, loop and unpaired regions are all important in interaction with host factors. The HCV IRES can directly recruit the 40S ribosomal subunit before association with eukaryotic translation factor (eIF)2 and eIF3 to form the 43S pre-initiation complex[35,65]. Structural and biochemical studies have indicated an unusually vast binding site for the ribosome encompassing domains II, III and IV and conformational changes in both the 40S ribosomal subunit and the IRES have been observed upon their interaction[66]. Binding between the IRES and the ribosome is thought to be mediated via ribosomal proteins although the role of RNA-RNA interaction cannot be excluded[67-69]. eIF3 can bind both the ribosome and junction domain IIIabc and domain IIIb. The ternary complex eIF2α-guanosine triphosphate (GTP)-the tRNA for the first methionine (MettRNAi) does not directly bind the IRES, rather it forms a ternary complex with eIF3 and the 40S ribosomal subunit to position MettRNAi directly onto the AUG codon in the P-site of the ribosome[70]. A number of non-canonical host factors are able to bind the HCV IRES and likely play a facilitating role in IRES translation. However, there is evidence for a critical role of the La autoantigen in IRES translation, by binding to and altering the conformation of the IRES to orchestrate assembly of the ribosomal complex[82,83]. NS: Non-structural.
- Citation: Chan SW. Establishment of chronic hepatitis C virus infection: Translational evasion of oxidative defence. World J Gastroenterol 2014; 20(11): 2785-2800
- URL: https://www.wjgnet.com/1007-9327/full/v20/i11/2785.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i11.2785